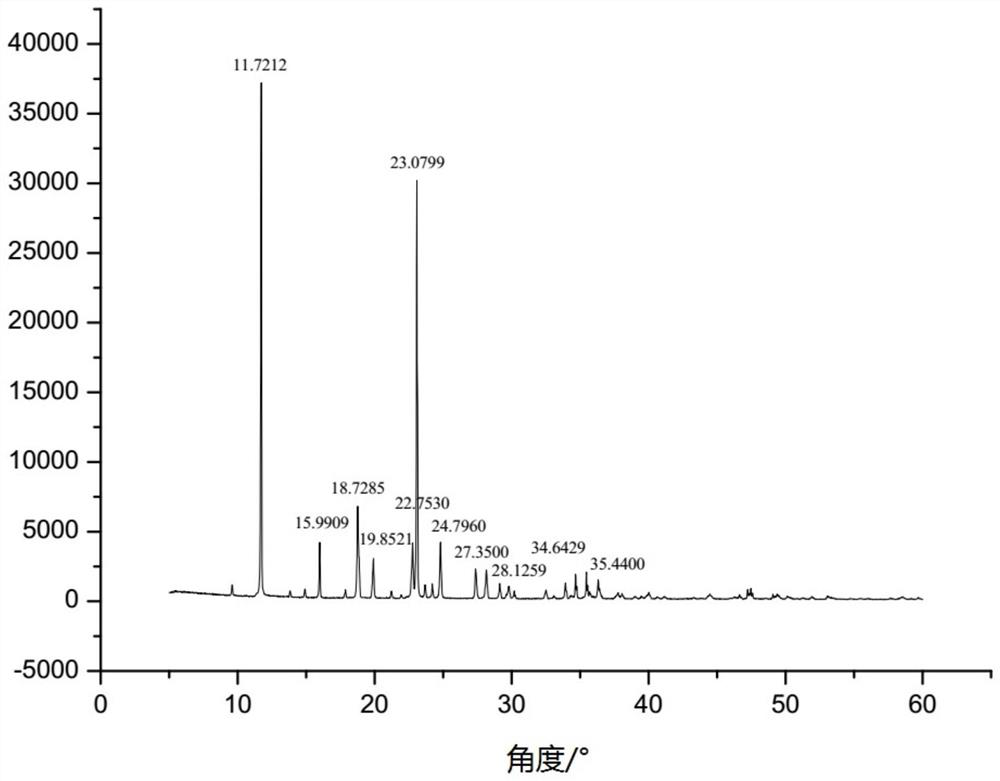
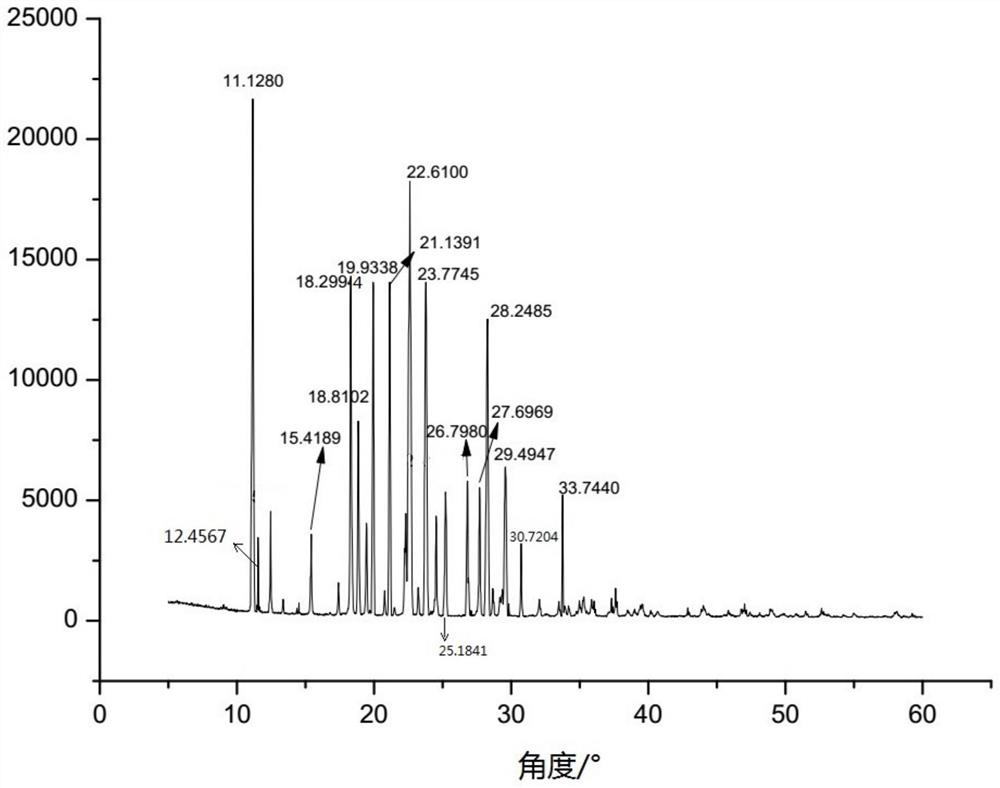
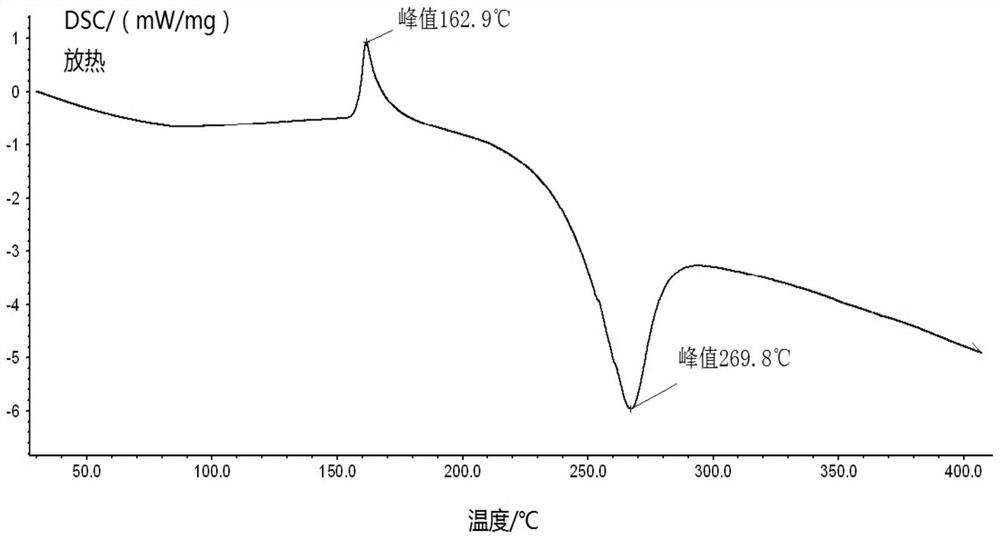
The New Indication of Trisofen Ceftriaxone Sodium Drug Preparation in the Treatment of Bacterial Endometritis
A ceftriaxone sodium and solvent technology, which is applied in the field of drug preparation, can solve problems such as hidden safety hazards, insufficient stability of ceftriaxone sodium, poor stability of ceftriaxone sodium preparations, etc., and achieve improved use safety and storage stability and outstanding solubility, reducing the risk of clinical sensitization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0126] The synthesis of embodiment 1-2 ceftriaxone sodium
[0127] The same as the synthesis process of Example 1-1, the only difference is: in step 1), 15gAlCl is added dropwise to the reaction system 3 -BF3-dimethyl carbonate solution [(wBF 3 )=18%] (wherein, AlCl 3 0.66g (0.005mol), BF 3 2.7g (0.04mol)), namely AlCl 3 -BF 3 AlCl in dimethyl carbonate composite catalyst 3 and BF 3 The molar ratio is 1:8.
[0128] The synthesis of embodiment 1-3 ceftriaxone sodium
[0129] Identical to the synthetic technique of embodiment 1-1, difference is only: in step 2), reaction solvent is by V 乙腈 :V 二氯甲烷 =5:1 mixed solvent, changed to acetonitrile, crystallization solvent is acetone.
Embodiment 1-4
[0130] The synthesis of embodiment 1-4 ceftriaxone sodium
[0131] The synthesis process is the same as in Example 1-1, except that in step 2), 192.7 g (0.55 mol) of AE-active ester, that is, the mass ratio of 7-ACT to AE-active ester is 1:1.0.
Embodiment 1-5
[0132] The synthesis of embodiment 1-5 ceftriaxone sodium
[0133] The synthesis process is the same as in Example 1-1, the only difference is that in step 2), the catalyst is changed from tri-n-butylamine and pyridine to triethylamine of the same molar amount. The color of the crude product of ceftriaxone sodium obtained is light yellow.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com