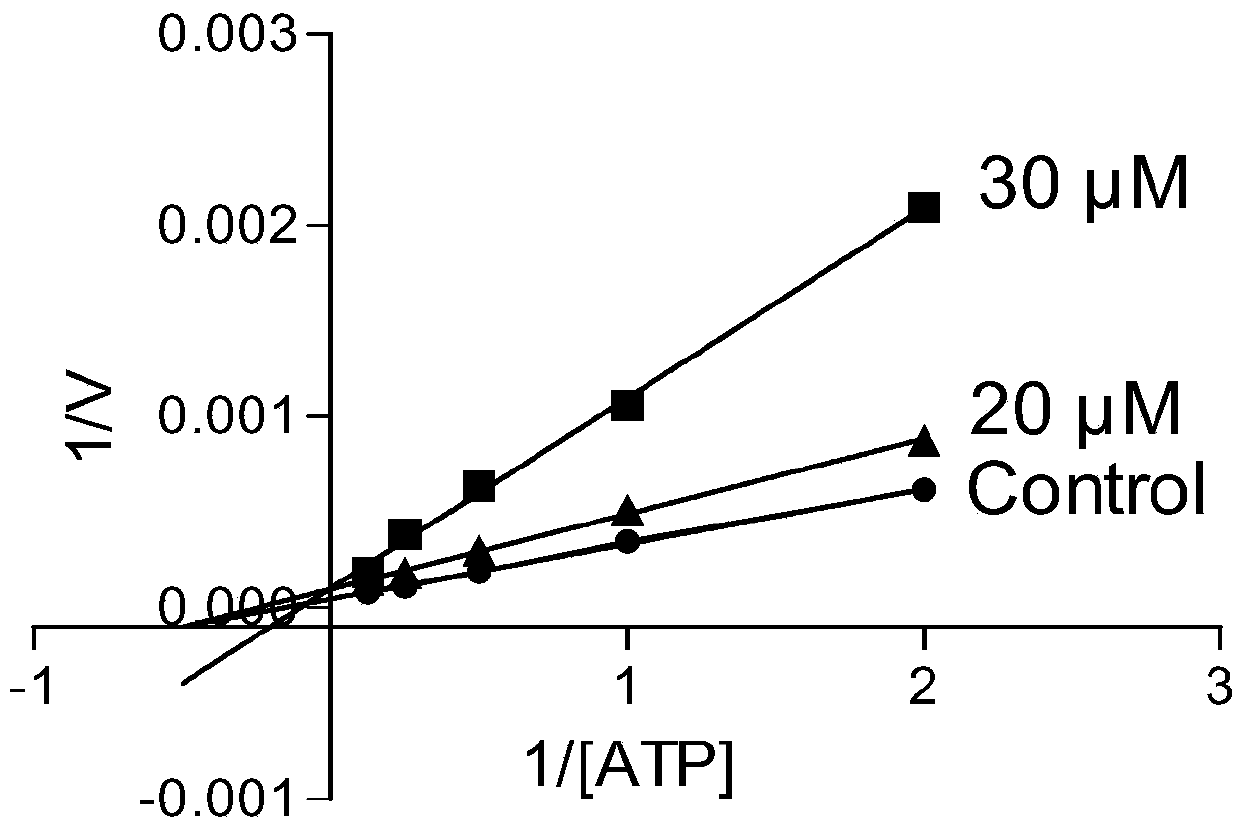
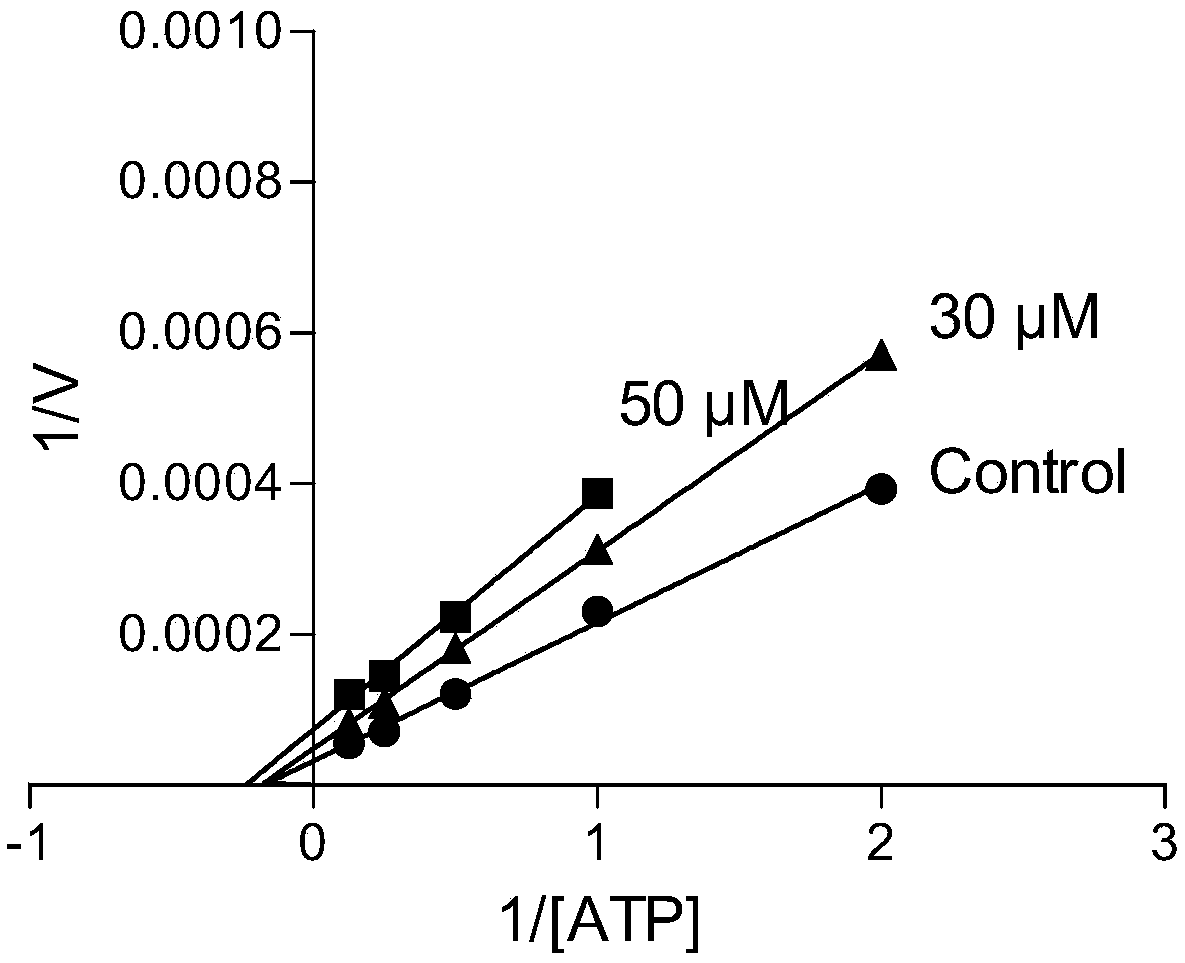
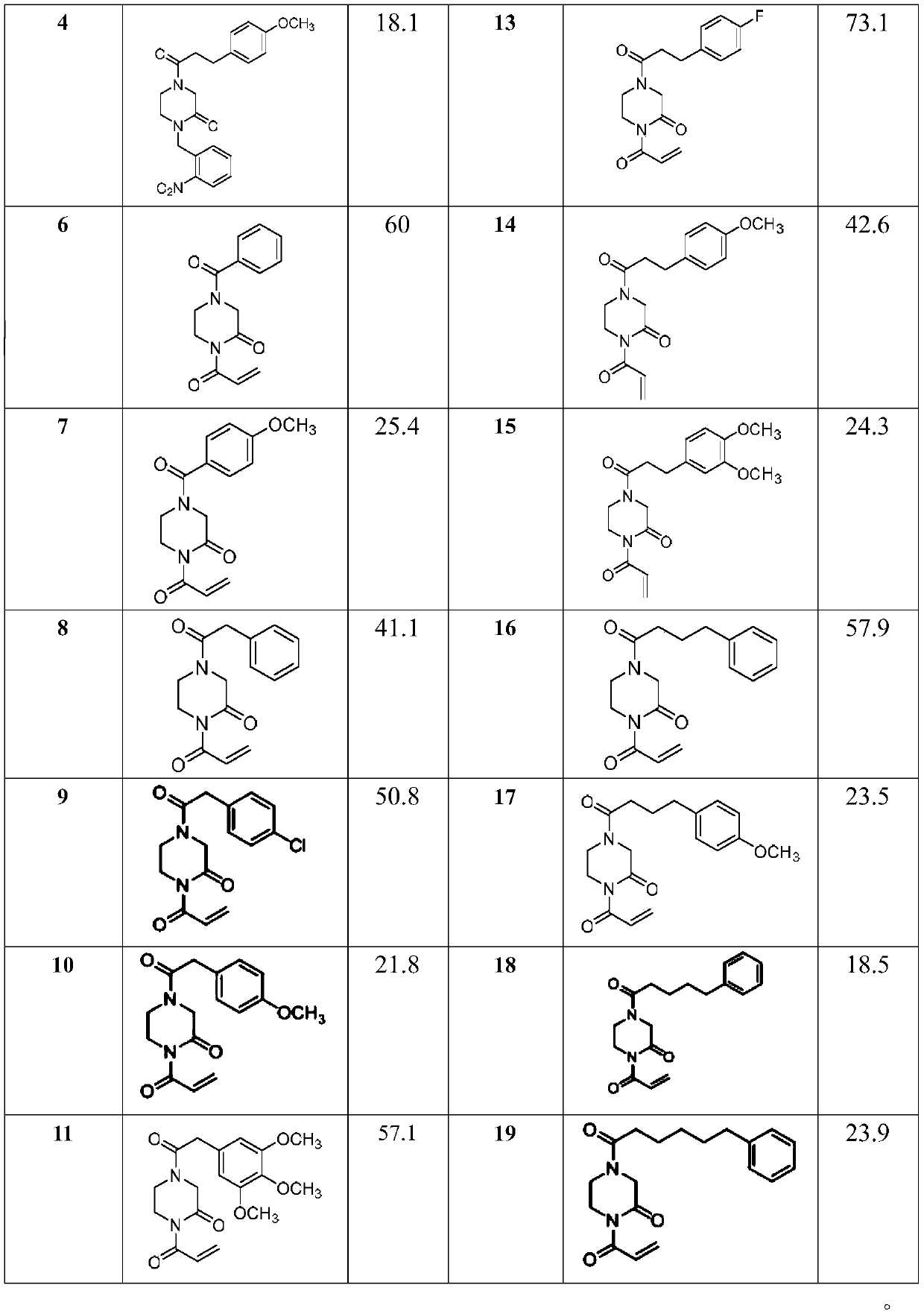
1,4-disubstituted-2-piperazinone compounds and pharmaceutical use thereof
A disubstituted, piperazinone technology, applied in 1,4-disubstituted-2-piperazinone compounds and its application in medicine, can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of 1-(2-nitrobenzyl)-4-(3-phenylpropionyl)piperazin-2-one (3), its structural formula is as follows:
[0023]
[0024] The first step: preparation of tert-butyl-4-(2-nitrobenzyl)-3-oxo-piperazine-1-carboxylate (1)
[0025]
[0026] Add 4-Boc-piperazinone (2.0g, 0.01mol), 2-nitrobenzyl bromide (2.4g, 0.01mol), potassium carbonate (2.7g, 0.02mol), acetone (100mL) to the reaction flask, and reflux React overnight. Dilute with ethyl acetate, wash with 1 mol / L aqueous hydrochloric acid, saturated aqueous sodium bicarbonate, and saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure to obtain a crude product, which is subjected to silica gel column chromatography (PE:EA=40:60) to obtain 1.5 g of a light yellow solid with a yield of 45%. 1 H NMR (400MHz, CDCl 3 )δppm 8.04(d,J=8.1Hz,1H),7.62(t,J=7.6Hz,1H),7.46(t,J=7.8Hz,1H),7.37(d,J=7.9Hz,1H), 5.00(s, 2H), 4.21(s, 2H), 3.69(t, J=5.5Hz, 2H), 3.36(t, J=5.4Hz,...
Embodiment 2
[0033] Embodiment 2: Preparation of 4-(3-(4-methoxyphenyl)propionyl)-1-(2-nitrobenzyl)piperazin-2-one (4), its structural formula is as follows:
[0034]
[0035] The preparation method is the same as that of the above-mentioned compound 3, and 4-methoxyphenylpropionic acid is used instead of phenylpropionic acid. A light yellow oil was obtained with a yield of 90%. ESI-MS m / z:398.2[M+H] + , 1 H NMR (400MHz, CDCl 3 )δppm 8.03(dd, J=12.1,8.1Hz,1H),7.62(dd,J=8.3,7.0Hz,1H),7.46(t,J=8.0Hz,1H),7.33(dd, J=26.1, 7.8Hz, 1H), 7.12(t, J=8.8Hz, 2H), 6.82(t, J=9.1Hz, 2H), 4.94(s, 2H), 4.35(s, 1H), 4.12(s, 1H) ,3.84(t,J=5.5Hz,1H),3.81-3.75(m,3H),3.58(t,J=5.4Hz,1H),3.30(t,J=5.5Hz,1H),3.22(t, J=5.4Hz, 1H), 2.94(t, J=7.5Hz, 2H), 2.65-2.58(m, 2H).
Embodiment 3
[0036] Example 3: Preparation of 1-acryloyl-4-benzoylpiperazin-2-one (6), whose structural formula is as follows:
[0037]
[0038] The first step: preparation of 4-benzoylpiperazin-2-one (5)
[0039]
[0040] Add 2-piperazinone (300mg, 3mmol), benzoic acid (402mg, 3.3mmol), HBTU (1.7g, 4.5mmol), DIPEA (1.6mL, 9mmol), dichloromethane (20mL) into the reaction flask, room temperature Reaction 8h. Diluted with dichloromethane, washed with saturated brine, and dried over anhydrous sodium sulfate. Concentrate under reduced pressure to obtain a crude product, which is subjected to silica gel column chromatography (DCM:MeOH=95:5) to obtain 550 mg of a light yellow oil, with a yield of 90%, ESI-MS m / z: 259.1 [M+H] + .
[0041] The second step: preparation of 1-acryloyl-4-benzoylpiperazin-2-one (6)
[0042]
[0043] Add 5 (408mg, 2mmol), DIPEA (1.0mL, 6mmol), dichloromethane (20mL) into the reaction flask, add acryloyl chloride (600μL, 6mmol) under ice bath, remove the ice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com