Dexamethasone slow-release microsphere for injection in glass body
A technology of dexamethasone and sustained-release microspheres, which is applied in microcapsules, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. Inappropriate path and other problems, achieve good stability and reconstitution ability, facilitate clinical application, and reduce the effect of drug burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
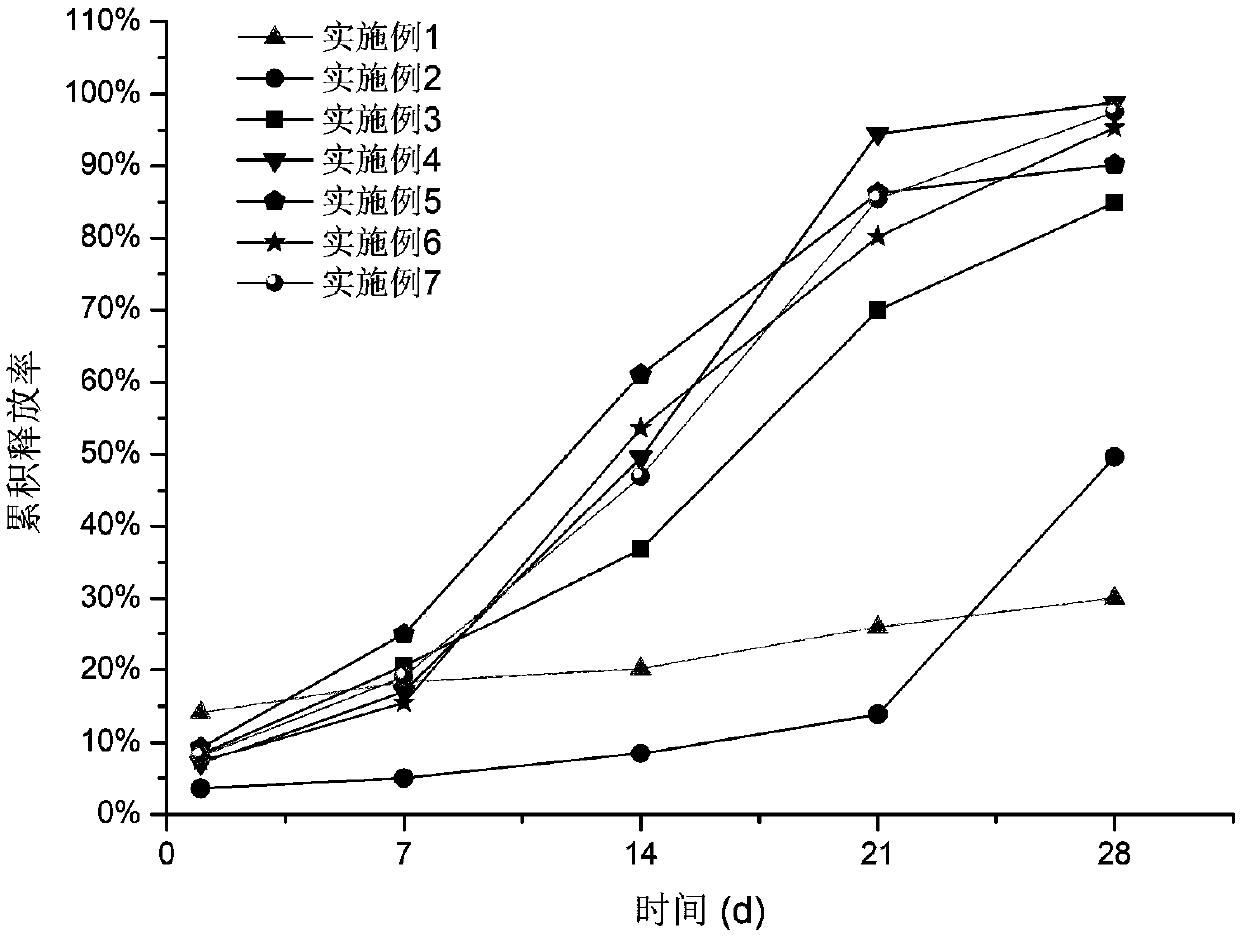
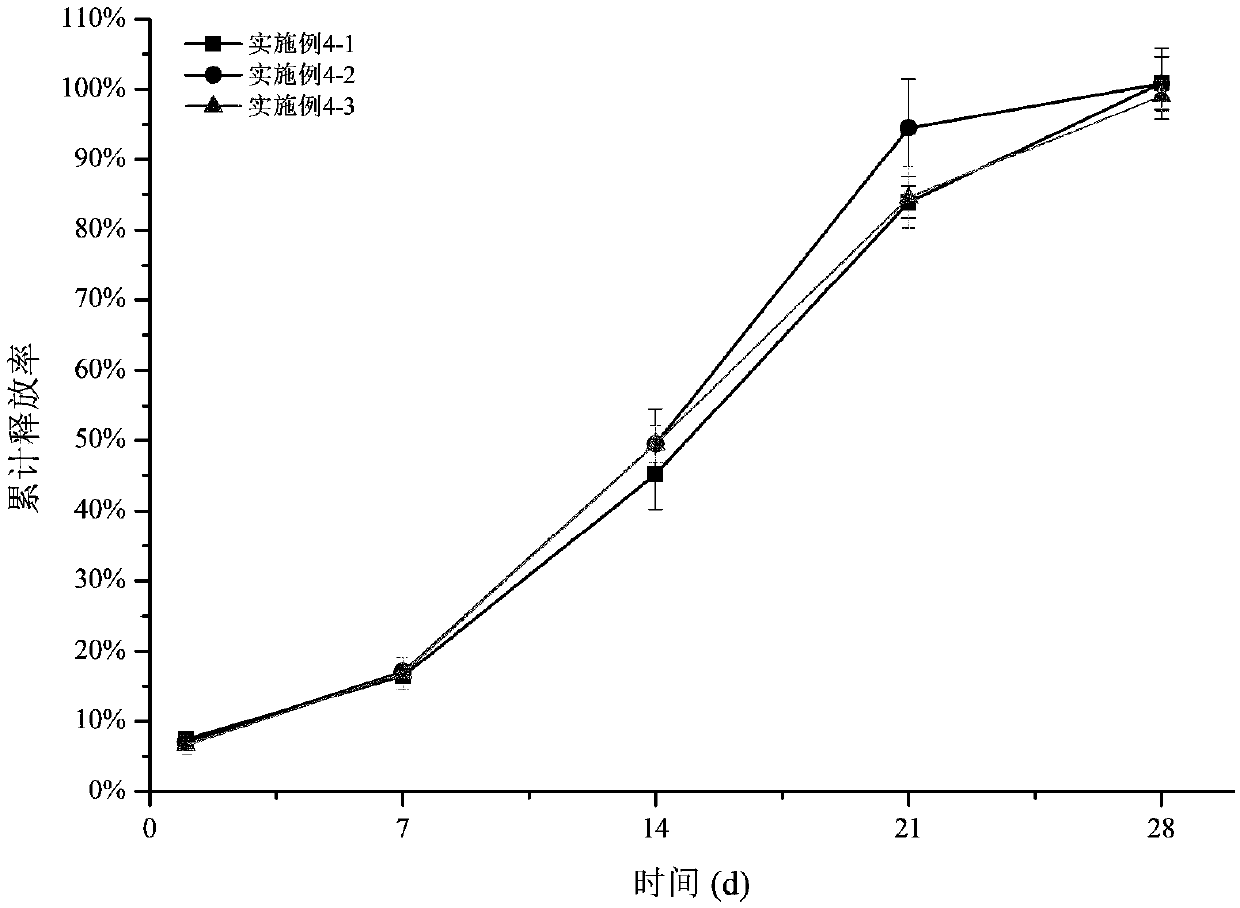
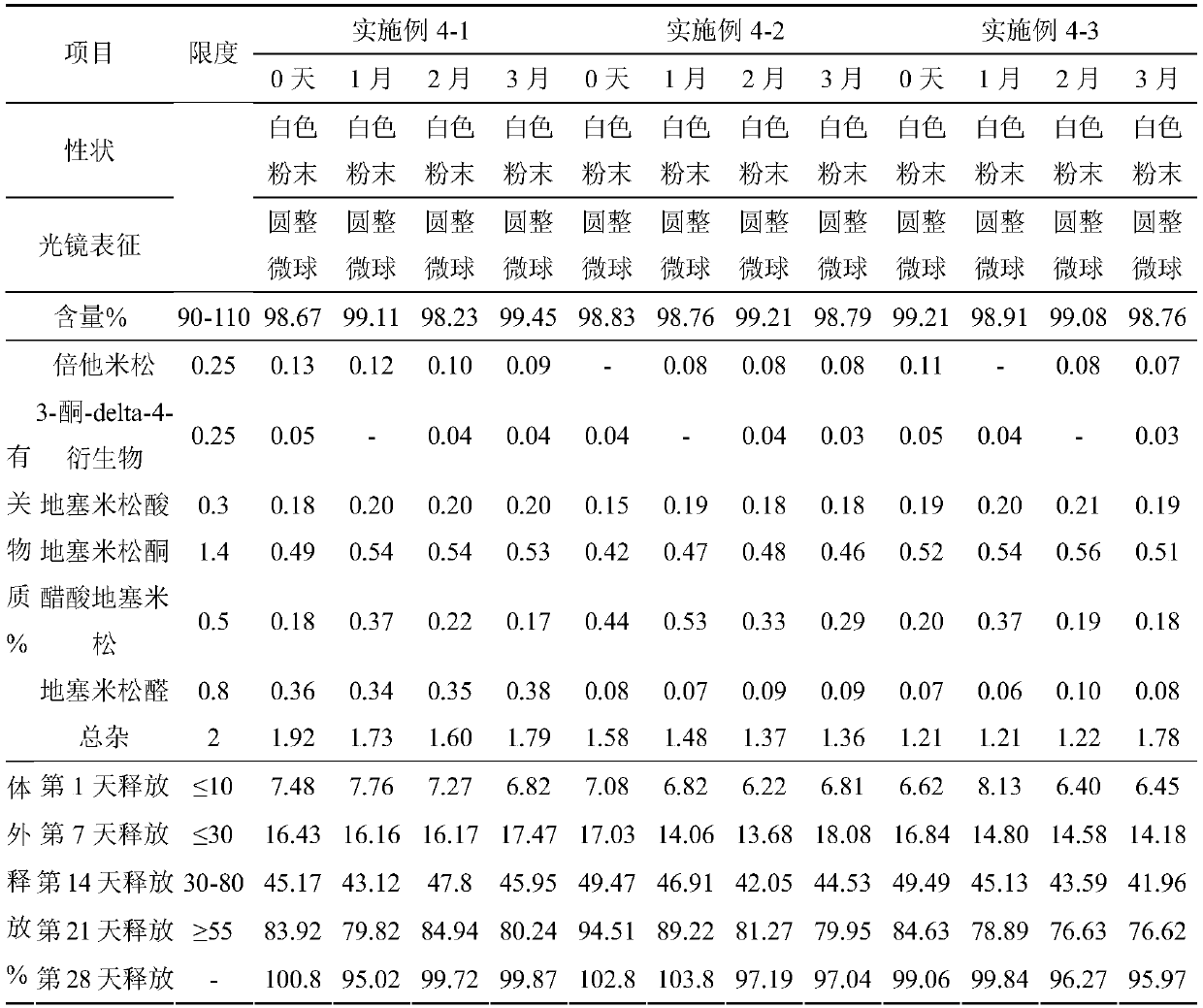
Embodiment 1
[0038] Dexamethasone 300mg
[0039] Ester-terminated glycolide-lactide copolymer ( RG 502) 400mg
[0040] Carboxyl-terminated glycolide-lactide copolymer ( RG 502 H) 0mg
[0041] (1) take by weighing the dexamethasone of formula quantity, ester group terminal glycolide lactide copolymer and carboxyl terminal glycolide lactide copolymer, be dissolved in 1.75ml dichloromethane and dimethyl sulfoxide volume ratio is In the mixed solvent of 5:2, make the inner oil phase for standby;
[0042] Weigh polyvinyl alcohol and add water, and prepare an aqueous solution with a mass concentration of 1% at 60-80° C., which is used as the external aqueous phase I for subsequent use;
[0043] Weigh polyvinyl alcohol and add water to prepare an aqueous solution with a mass concentration of 0.2% at 60-80°C, which will be used as the external aqueous phase II for later use;
[0044] (2) Add the above-mentioned inner oil phase into 300ml of outer water phase I, and emulsify at 1700rpm for...
Embodiment 2
[0049] Dexamethasone 300mg
[0050] Ester-terminated glycolide-lactide copolymer ( RG 502) 0mg
[0051] Carboxyl-terminated glycolide-lactide copolymer ( RG 502 H) 400mg
[0052] (1) take by weighing the dexamethasone of formula quantity, ester group terminal glycolide lactide copolymer and carboxyl terminal glycolide lactide copolymer, be dissolved in 1.75ml dichloromethane and dimethyl sulfoxide volume ratio is In the mixed solvent of 5:2, make the inner oil phase for standby;
[0053] Weigh polyvinyl alcohol and add water, and prepare an aqueous solution with a mass concentration of 1% at 60-80° C., which is used as the external aqueous phase I for subsequent use;
[0054] Weigh polyvinyl alcohol and add water to prepare an aqueous solution with a mass concentration of 0.2% at 60-80°C, which will be used as the external aqueous phase II for later use;
[0055] (2) Add the above-mentioned inner oil phase into 300ml of outer water phase I, and emulsify at 1700rpm for...
Embodiment 3
[0060] Dexamethasone 150mg
[0061] Ester-terminated glycolide-lactide copolymer ( RG 502) 250mg
[0062] Carboxyl-terminated glycolide-lactide copolymer ( RG 502 H) 250mg
[0063] (1) take by weighing the dexamethasone of formula quantity, ester group terminal glycolide lactide copolymer and carboxyl terminal glycolide lactide copolymer, be dissolved in 1.75ml dichloromethane and dimethyl sulfoxide volume ratio is In the mixed solvent of 5:2, make the inner oil phase for standby;
[0064] Weigh polyvinyl alcohol and add water, and prepare an aqueous solution with a mass concentration of 1% at 60-80° C., which is used as the external aqueous phase I for subsequent use;
[0065] Weigh polyvinyl alcohol and add water to prepare an aqueous solution with a mass concentration of 0.2% at 60-80°C, which will be used as the external aqueous phase II for later use;
[0066] (2) Add the above-mentioned inner oil phase into 300ml of outer water phase I, and emulsify at 2500rpm f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com