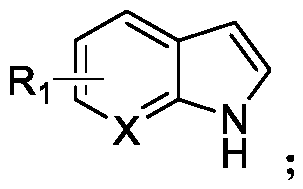
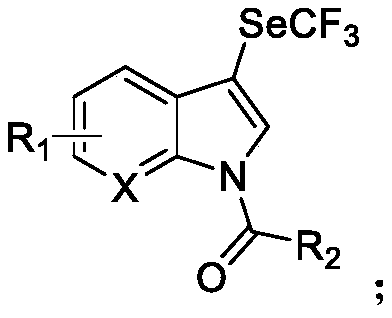
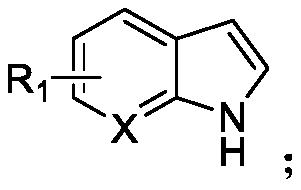
N-acylated trifluoro-methylseleno bifunctionalization method for indole derivative
A technology of indole derivatives and acylated trifluoromethane, which is applied in the direction of organic chemistry, can solve the problems of application limitations, high toxicity, and corrosiveness, and achieve the effects of simple operation, mild conditions, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Under nitrogen protection, the [Me 4 N][SeCF 3 ] (133mg, 0.6mmol) and the oxidant 3,3'-dichlorobenzoyl peroxide (137mg, 0.44mmol) were dissolved in 1mL of acetonitrile, and 1mL of indole (23.4mg, 0.2mmol) was added dropwise at 25°C acetonitrile solution, and reacted at 25°C for 16 hours. After the reaction was completed, two drops of water were dropped to quench, and then the solvent was removed by rotary evaporation, and petroleum ether and ethyl acetate (20:1) were used as eluents to obtain the acylated trifluoromethylselenide product by column chromatography: N- m-Chlorobenzoyl-3-trifluoromethaneselenoindole (yellow solid 56.3 mg, yield 70%), 1 H NMR (500MHz, CDCl 3 )δ8.38(d, J=7.1Hz, 1H), 7.77-7.75(m, 2H), 7.66-7.62(m, 3H), 7.52(t, J=7.8Hz, 1H), 7.50-7.45(m ,2H); 19 FNMR (471MHz, CDCl 3 )δ-35.9(s,3F).
Embodiment 2
[0052] Under nitrogen protection, the AgSeCF 3 (38mg, 0.6mmol) and the oxidizing agent 3,3'-dichlorobenzoyl peroxide (124mg, 0.4mmol) were dissolved in 1mL of acetonitrile, cooled to -40°C and 1mL of 2-methylindole (26.2mg , 0.2 mmol) in acetonitrile solution, and reacted at -40°C for 48 hours. After the reaction was completed, two drops of water were dropped to quench, and then the solvent was removed by rotary evaporation, and petroleum ether and ethyl acetate (20:1) were used as eluents to obtain the acylated trifluoromethylselenide product by column chromatography: N- m-chlorobenzoyl-2-methyl-3-trifluoromethylselenoindole (yellow solid 25.8 mg, yield 31%), 1 H NMR (500MHz, CDCl 3 )δ7.77(t, J=1.7Hz,1H),7.70(d,J=7.9,1H),7.67-7.64(dm,J=7.9,1H),7.60-7.58(dt,J=7.8,1.2 ,1H),7.46(t,J=7.9,1H),7.29(t,J=7.8,1H),7.15(t,J=7.2,1H),7.00(d,J=8.4,1H),2.65( s,3H); 19 F NMR (471MHz, CDCl 3 )δ-35.9(s,3F).
Embodiment 3
[0054] Under nitrogen protection, the [Et 4 N][SeCF 3 ] (168mg, 0.6mmol) and the oxidant 3,3'-dichlorobenzoyl peroxide (124mg, 0.4mmol) were dissolved in 1mL DMF, and 1mL 4-fluoroindole (27.0mg, 0.2 mmol) in DMF and reacted at 25°C for 24 hours. After the reaction was completed, two drops of water were dropped to quench, and then the solvent was removed by rotary evaporation, and petroleum ether and ethyl acetate (20:1) were used as eluents to obtain the acylated trifluoromethylselenide product by column chromatography: N- m-chlorobenzoyl-3-trifluoromethylselenyl-4-fluoroindole (yellow solid 43.1mg, yield 51%), 1 H NMR (500MHz, CDCl 3 )δ8.20(d, J=8.4Hz,1H),7.76(t,J=1.8,1H),7.67-7.65(dm,J=8.0,1H),7.63-7.61(dt,J=7.7,1.3 ,1H),7.55(s,1H),7.53(t,J=7.9,1H),7.43-7.39(m,1H),7.12-7.08(m,1H); 19 F NMR (471MHz, CDCl 3 ) δ-36.8(s, 3F), δ-123.7(m, 1F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com