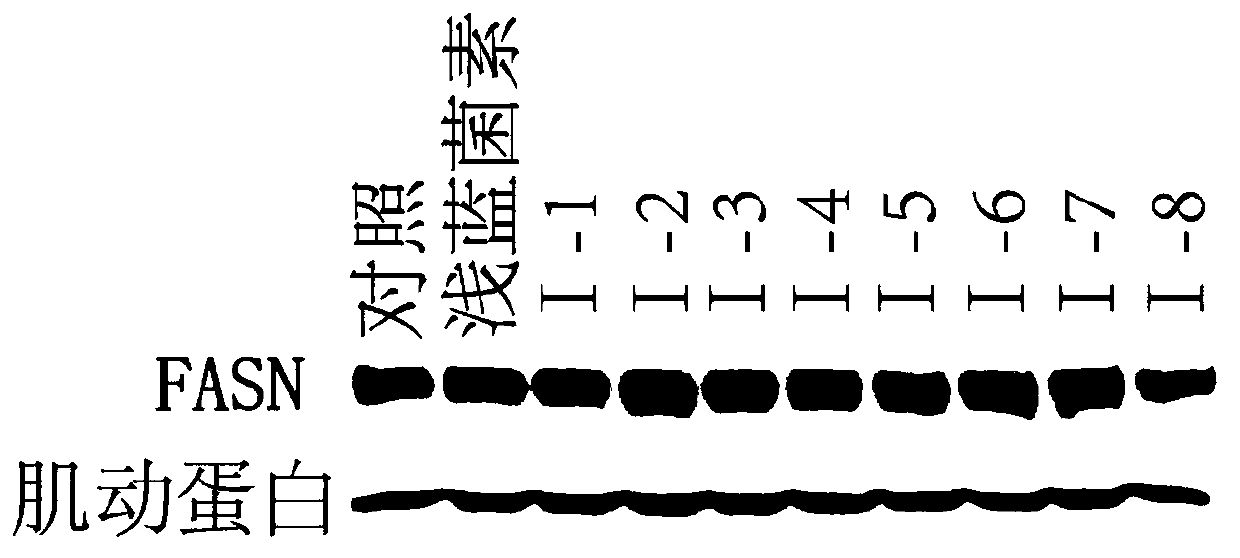
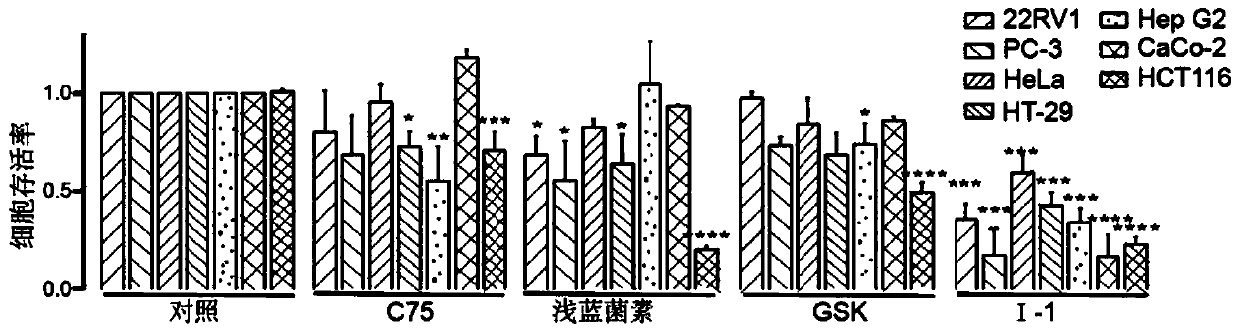
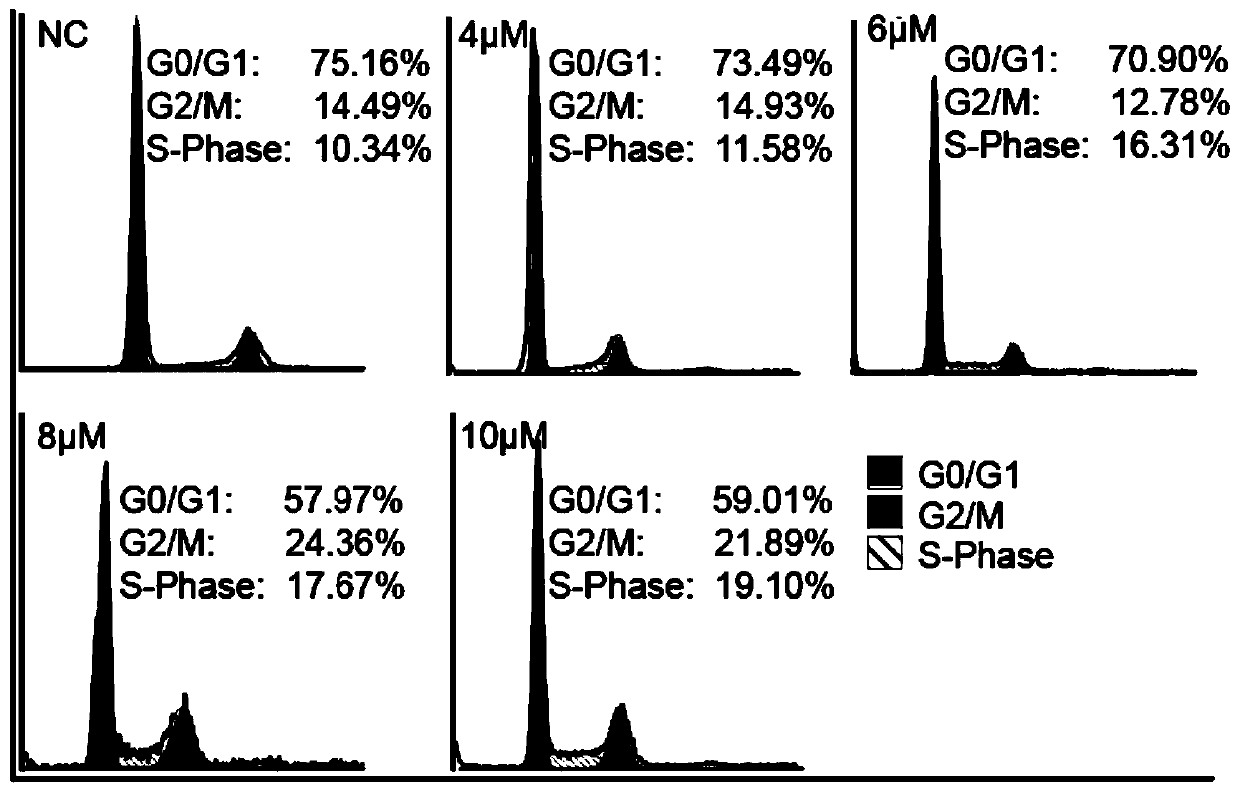
Fat synthetase inhibitor and applications thereof
A technology of fatty acid synthase and compound, which is applied in the field of medicine and biology, can solve the problems of tumor cell apoptosis and unclear tumor mechanism, and achieve the effect of promoting tumor cell apoptosis, important clinical application prospects, and inhibiting tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 formula I-1 compound
[0045]
[0046] The specific synthesis method is as follows:
[0047] (1) Synthesis of intermediate 1 compound 5-(4-bromophenylamino)-5-oxopentanoic acid:
[0048] Add 5-ethoxy-5-oxopentanoic acid (2g, 12.5mM) to a 100mL single-necked bottle, and dichloromethane (30mL), cool to 0°C in an ice bath, and slowly add thionyl chloride (3g, 25mM). After reaching room temperature, heat to reflux. The reaction solution was quenched and spin-dried to obtain 1 g of intermediate 1 product with a yield of 44.9%;
[0049] (2) Synthesis of intermediate 2 compound 5-(4-bromophenylamino)-5-carbonylpentanoic acid ethyl ester:
[0050] Add p-bromoaniline (1.15g, 6.7mmol), dichloromethane (15mL), and triethylamine (0.85g, 8.4mmol) to a 100mL single-necked bottle, cool down to 0°C, and slowly add intermediate 1 (1g, 5.6mmol) at room temperature and stirred overnight, TLC showed that the reaction was complete, the reaction solution ...
Embodiment 2
[0056] The synthesis of embodiment 2 formula I-2 compound
[0057]
[0058] (1) Synthesis of 4-((tert-butoxycarbonyl) amino) butanoic acid:
[0059] Add 4-aminobutyric acid (2g, 19.4mmol, 1.0eq), THF (15mL), water (15mL), sodium hydroxide (1.6g, 38.8mmol) into a 100mL single-necked bottle, cool to 0°C, and slowly drop Plus (Boc) 2 O (5.1g, 23.3mmol), stirred at 25°C for 12hrs after dropping. After the reaction was complete, THF was removed by rotary evaporation, 15 mL of water was added, and excess (Boc) was extracted by ethyl acetate (10 mL). 2 O, the aqueous phase was adjusted to pH 2-3 with citric acid or hydrochloric acid, extracted with ethyl acetate (15 mL), and the organic phase was dried with anhydrous sodium sulfate and spin-dried to obtain 3.5 g of product with a yield of 88%.
[0060] (2) Synthesis of N-(4-bromophenyl)-4-((tert-butoxycarbonyl)amino)butanamide:
[0061] Add p-bromoaniline (1g, 5.8mmol), dichloromethane (15mL), and triethylamine (0.76g, 7.4mmol...
Embodiment 3
[0067] The synthesis of embodiment 3 formula I-3 compound
[0068] The structure of compound 1-3 is shown below:
[0069]
[0070] Dissolve I-2 compound (0.6mmol) in dichloromethane (5mL), cool to -20°C, slowly add BBr 3 (0.9 mmol). It was then stirred at room temperature for 3 hours. TLC showed the reaction was complete. Add water (15mL) to quench, DCM (15mL) to extract, after the organic phase is dried, the organic phase is dried with anhydrous sodium sulfate, filters, after spin-drying, obtain crude product, silica gel column chromatography (petroleum ether:ethyl acetate=100:1 to 3:1) to obtain 200 mg of I-3 compound with a yield of 69%.
[0071] 1 H-NMR (DMSO, 400MHz) δ: 1.65(m,2H), 2.31(t,2H), 3.05(t,2H), 4.13(d,2H), 6.22(t,1H), 6.46(t,1H ), 6.74(t,1H), 7.20(m,2H), 7.52(m,2H), 7.60(m,2H), 10.05(s,1H), 10.12(s,1H). Mass spectrum (MS+): 485,487, m / z: [M+2, M+4].
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com