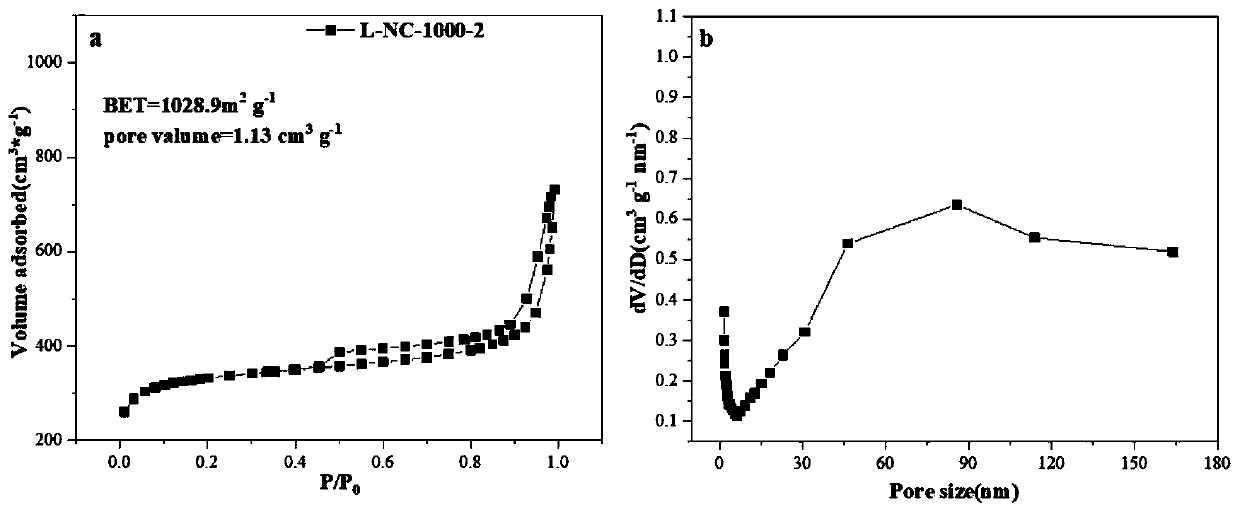
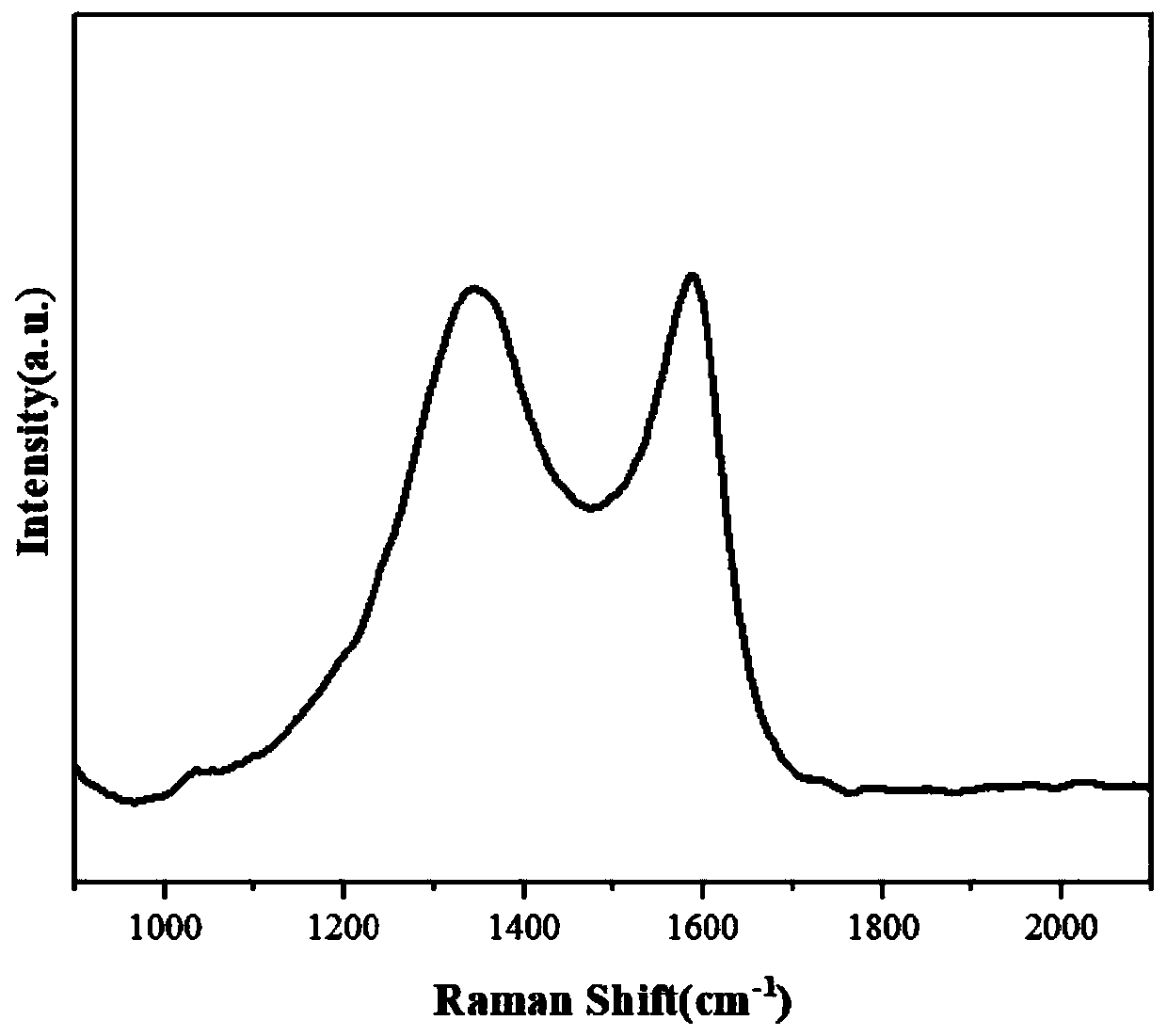
Preparation method of ZIF-8-derived N,S-co-doped non-metallic carbon-based nano-oxygen reduction electrocatalyst
A ZIF-8, carbon-based nanotechnology, applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, to achieve the effect of improving electrical conductivity, low cost, and good long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] Its preparation method specifically comprises the following steps:
[0031] 1.1. Preparation of ZIF-8:
[0032] Weigh 1.68g of zinc nitrate hexahydrate solid, dissolve it in 80mL of methanol solution, and ultrasonically dissolve it completely to obtain zinc nitrate hexahydrate solution, and weigh 3.7g of 2-methylimidazole solid, dissolve it in 80mL of methanol In the solution, ultrasound was used to completely dissolve it to obtain a zinc nitrate hexahydrate solution and a 2-methylimidazole solution. The above two solutions were mixed and stirred, and reacted at room temperature for 24 hours to obtain a ZIF-8 solution; the obtained ZIF-8 The solution is centrifuged in a centrifuge to obtain a ZIF-8 solid containing methanol, zinc nitrate, and 2-methylimidazole; the obtained ZIF-8 solid containing methanol, zinc nitrate, and 2-methylimidazole is washed with methanol under ultrasonic 3-5 times, vacuum-dried at 60°C for 12 hours to obtain a ZIF-8 solid containing 2-methyl...
specific Embodiment 1
[0045] 1.1. Preparation of ZIF-8:
[0046] Weigh 1.68g of zinc nitrate hexahydrate solid, dissolve it in 80mL of methanol solution, and ultrasonically dissolve it completely to obtain zinc nitrate hexahydrate solution, and weigh 3.7g of 2-methylimidazole solid, dissolve it in 80mL of methanol In the solution, ultrasound was used to completely dissolve it to obtain a zinc nitrate hexahydrate solution and a 2-methylimidazole solution. The above two solutions were mixed and stirred, and reacted at room temperature for 24 hours to obtain a ZIF-8 solution; the obtained ZIF-8 The solution is centrifuged in a centrifuge to obtain a ZIF-8 solid containing methanol, zinc nitrate, and 2-methylimidazole; the obtained ZIF-8 solid containing methanol, zinc nitrate, and 2-methylimidazole is washed with methanol under ultrasonic 3-5 times, vacuum-dried at 60°C for 12 hours to obtain a ZIF-8 solid containing 2-methylimidazole; dissolve the above-mentioned ZIF-8 solid containing 2-methylimidaz...
specific Embodiment 2
[0052] 2.1. Preparation of ZIF-8:
[0053] Prepared according to the method and conditions of step 1.1 in Example 1;
[0054] 2.2. Preparation of catalyst precursor:
[0055] Weigh 400 mg of the above ZIF-8, 170 mg of 4-(2-thienyl) pyrimidine-2-thiol, and 87 mg of triethylamine, and add the above-mentioned substances to a mixture of 200 mL of methanol and dichloromethane (V:V=1:1). In the mixed solution, stir for 24 h, centrifuge, wash with methanol three times, and dry in vacuum; obtain the ZIF-8 catalyst precursor material containing heteroatom small molecules;
[0056] 2.3. Preparation of N, S-co-doped non-metallic carbon-based nanocatalysts:
[0057] Prepared according to the method and conditions of step 1.3 in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com