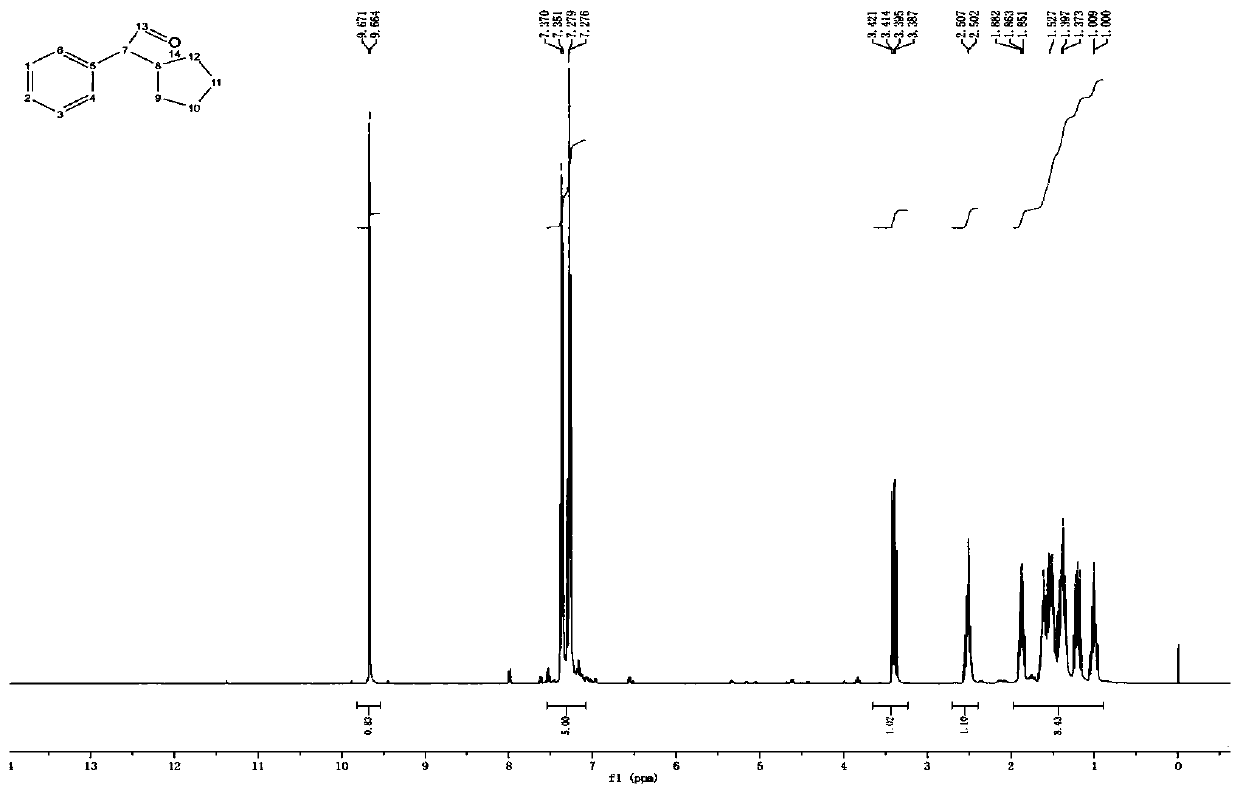
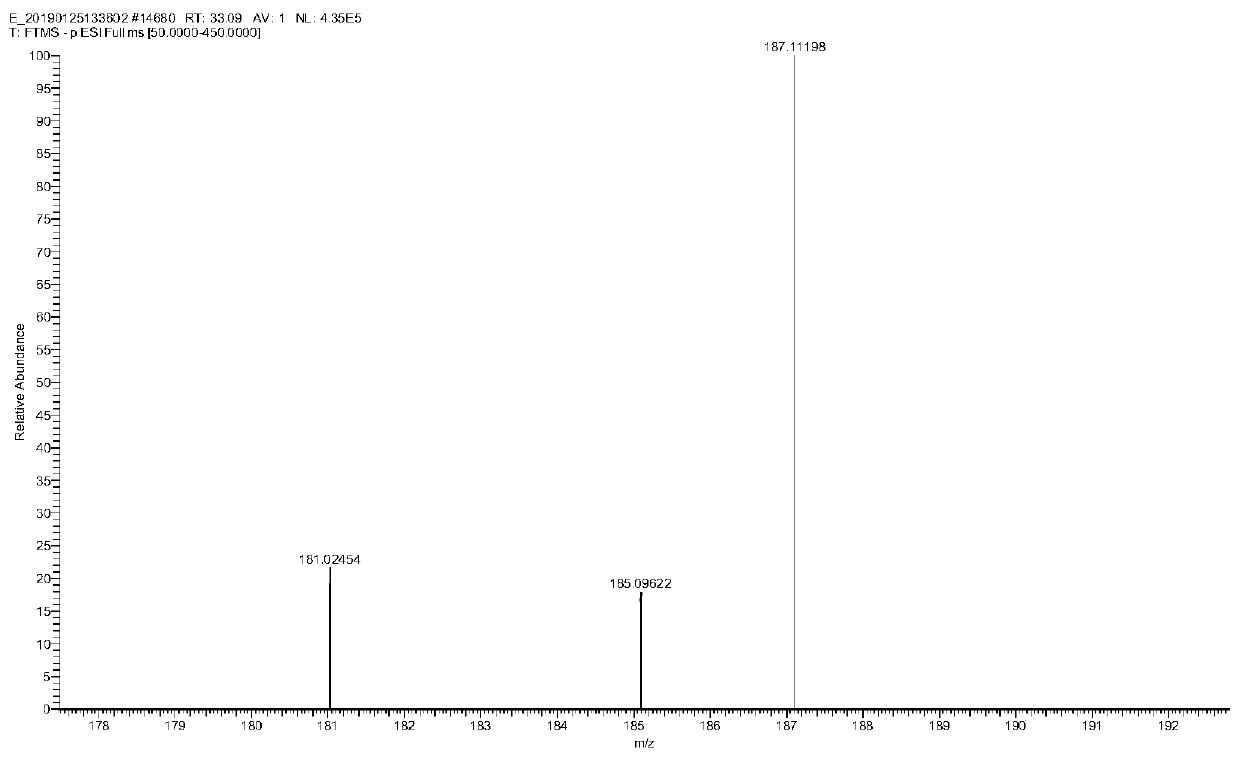
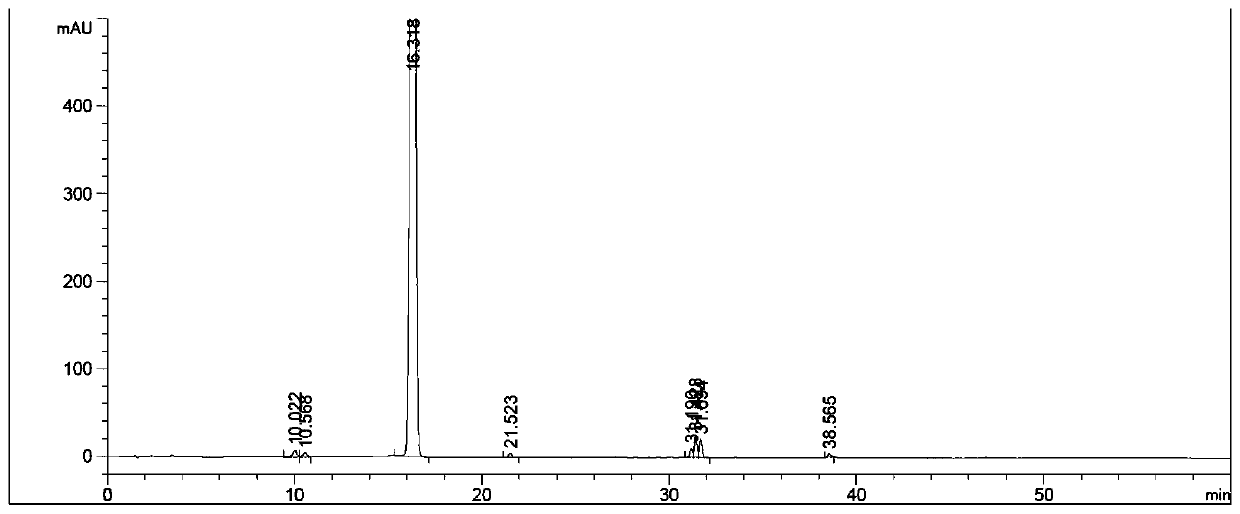
Method for preparing cyclopentyl phenyl acetaldehyde
A technology of cyclopentylphenyl and cyclopentylphenyl oxirane, which is applied in the field of preparation of cyclopentylphenylacetaldehyde, can solve problems affecting the quality of penhyclidine hydrochloride, etc., and achieve easy operation and improved The effect of quality standards and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of cyclopentylphenylacetaldehyde, comprising the following steps:
[0039] (1) Weigh 20 g of cyclopentylphenyl oxirane, add it to the reaction flask, add 200 g of tetrahydrofuran, stir and dissolve, then add 16.7 g of indium trichloride;
[0040] (2) Stir, react at 25-30°C for 3 hours, add 200g of water after the reaction to quench the reaction, add ethyl acetate for extraction, 100g each time, combine the organic phases and add 50g of anhydrous sodium sulfate to dry for 1 hour, after drying, filter Sodium sulfate was removed and concentrated to obtain 12.4 g of an oily substance.
[0041] (3) Add 20 g of ethyl acetate to dissolve the obtained oil, add 25 g of silica gel, stir evenly, concentrate to dryness, and set aside. Weigh 300g of silica gel, add hexane and stir evenly, pour it into a glass column for sedimentation, after the column is packed, add silica gel with sample, then add a mixed solution of hexane and ethyl acetate to elute, the hexa...
Embodiment 2
[0050] A preparation method of cyclopentylphenylacetaldehyde, comprising the following steps:
[0051] (1) Weigh 25 g of cyclopentylphenyl oxirane, add it into a reaction flask, add 300 g of tetrahydrofuran, stir to dissolve, and then add 18.5 g of indium trichloride.
[0052] (2) Stir, react at 20-25°C for 4 hours, add 250g of water after the reaction to quench the reaction, then extract with ethyl acetate, 125g each time, combine the organic phases and add 50g of anhydrous sodium sulfate to dry for 2 hours, after drying, filter Sodium sulfate was removed and concentrated to obtain 16.3 g of oily substance.
[0053] (3) Add 30 g of ethyl acetate to dissolve the obtained oil, add 32 g of silica gel, stir evenly, concentrate to dryness, and set aside. Weigh 350g of silica gel, add hexane and stir evenly, pour it into a glass column to settle, after the column is packed, add silica gel with sample, then add a mixed solution of hexane and ethyl acetate for elution, the hexane an...
Embodiment 3
[0055] A preparation method of cyclopentylphenylacetaldehyde, comprising the following steps:
[0056] (1) Weigh 10 g of cyclopentylphenyl oxirane, add it into a reaction flask, add 100 g of tetrahydrofuran, stir to dissolve, and then add 7.1 g of indium trichloride.
[0057] (2) Stir, react at 25-30°C for 4 hours, add 100g of water after the reaction to quench the reaction, then extract with ethyl acetate, 50g each time, combine the organic phases and add 30g of anhydrous sodium sulfate to dry for 1 hour, after drying, filter Sodium sulfate was removed and concentrated to obtain 5.9 g of oil.
[0058] (3) Add 10 g of ethyl acetate to dissolve the obtained oil, add 12 g of silica gel, stir evenly, concentrate to dryness, and set aside. Weigh 120g of silica gel, add hexane and stir evenly, pour into a glass column for sedimentation, after the column is packed, add silica gel with sample, then add a mixed solution of hexane and ethyl acetate for elution, the hexane and ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com