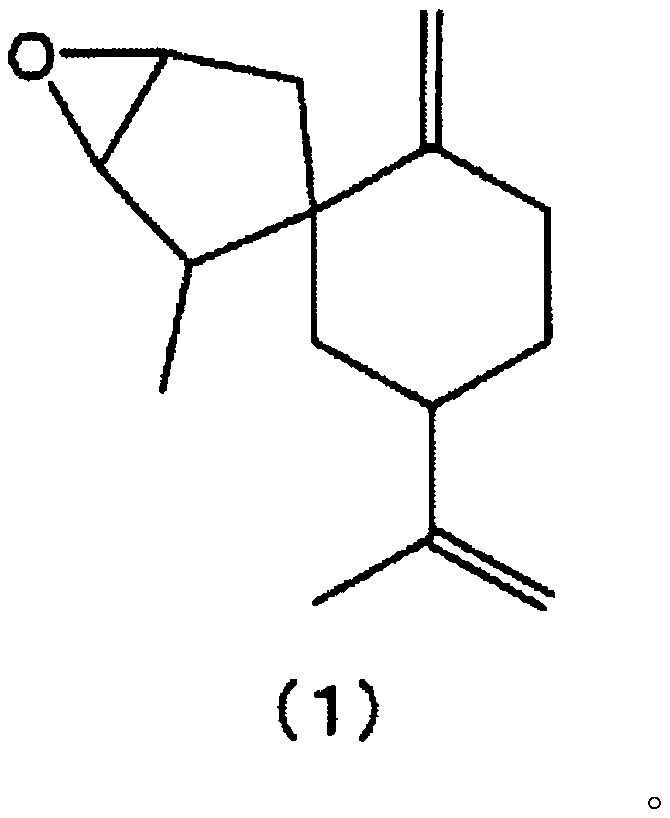
Novel spirosesquiterpene compound, flavoring composition and food/drink containing said compound, and method for producing said food/drink
A technology for manufacturing methods and compositions, applied in the field of spirocyclic sesquiterpene compounds, capable of solving problems such as insufficient response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (Example 1) Production of the compound of the present invention
[0070] The production method of the compound of the present invention will be specifically described using the following production examples, but the production method of the compound of the present invention is not limited to these production examples.
[0071] It should be noted that, unless otherwise specified, the input of substrates and solvents was performed under a nitrogen stream, and the post-treatment of the reaction solution and purification of the crude product were performed in air. In addition, the purity of the compounds obtained in the following production examples was determined by NMR analysis or gas chromatography analysis.
[0072] In the following production examples, the apparatus and conditions used for the measurement of physical properties are as follows.
[0073] NMR measurement device: AVANCEIII 500 (manufactured by Bruker BioSpin)
[0074] Gas chromatography measurement devic...
manufacture example 1
[0078] (Production Example 1) Synthesis of (3aS, 6S, 7aS)-6-(1-propen-2-yl)hexahydroisobenzofuran-1(3H)-one (4) (Eq.1)
[0079] Eq.1
[0080]
[0081] In a 100mL four-necked round-bottom flask, add palladium acetate (0.72g, 3.2mmol, 0.08 equivalents), dehydrated dichloromethane (40.0mL), 1,4-diphenylphosphinobutane (dppb) (1.37g, 3.2 mmol) and (S)-perillyl alcohol (6.09 g, 40.0 mmol) were stirred at room temperature to prepare a matrix solution. After adding 23.0 mL of the matrix solution to a 100 mL autoclave, the interior was pressurized to 5.0 MPa with a mixed gas containing equimolar amounts of hydrogen and carbon monoxide, and stirred at an external temperature of 110° C. for 9 hours. The autoclave was cooled to room temperature, taking care to release gas components. After filtering the insoluble matter, the reaction solution was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (eluent: toluene / ethyl acetat...
manufacture example 2
[0087] (Production Example 2) Synthesis of (3aS, 6S, 7aR)-7a-allyl-6-(1-propen-2-yl)hexahydroisobenzofuran-1(3H)-one (5) (Eq .2)
[0088] Eq.2
[0089]
[0090] Into a 1000 mL four-necked round bottom flask, diisopropylamine (14.3 mL, 102.0 mmol) and dehydrated tetrahydrofuran (THF) (130 mL) were charged, and cooled in a dry ice-acetone bath. Next, an n-hexane solution (concentration: 1.55 mol / L, 57.9 mL, 89.7 mmol) of n-butyllithium (n-BuLi) was added dropwise using a dropping funnel at such a rate that the internal temperature was kept below -50°C. The resulting solution was warmed up to 0° C. in an ice bath, and stirred at the same temperature for 30 minutes. The resulting pale yellow solution was cooled with a dry ice-acetone bath. Dehydrated THF ( 65mL) solution. After the dropwise addition, dehydrated THF (10 mL) was added to the dropping funnel, and added dropwise to the flask. In the same dropping funnel, a solution of allyl bromide (11.84 g, 97.9 mmol) in deh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com