Prostate cancer pet diagnostic reagent 68 ga-dota-ancp-psma and its preparation method and application
A DOTA-ANCP-PSMA, 68ga-dota-ancp-psma technology, applied in the field of medicine, can solve the problems of not being patented, not suitable for targeted therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment provides a prostate cancer PET diagnostic reagent 68 The preparation method of Ga-DOTA-ANCP-PSMA, described method comprises the steps:
[0042] (S1) Synthesizing the precursor compound DOTA-ANCP-PSMA by solid phase synthesis;
[0043] Preparation of compound 3
[0044] The synthetic route is shown below. Using Fmoc-Lys(Dde)-Wang Resin (compound 1) as the starting material, weigh 3g of the raw material with a substitution degree of 0.3mmol / g, add it to the reactor, add DMF, and soak for 30min. Then drain the DMF, add 3 times the volume of 20% Pip / DMF, and fill with nitrogen gas to remove Fmoc, react for 30 minutes, drain the 20% Pip / DMF, wash with DMF 5 times, and the ninhydrin detection shows dark blue. Add N,N'-succinimidyl carbonate (DSC), N,N-diisopropylethylamine (DIPEA) and 4-dimethylaminopyridine (DMAP) in proportion, and the input ratio is resin:DSC: DIPEA:DMAP=1:6:12:1, add an appropriate amount of DMF, and react for 1 h under the protection ...
Embodiment 2
[0058] In vitro stability assay:
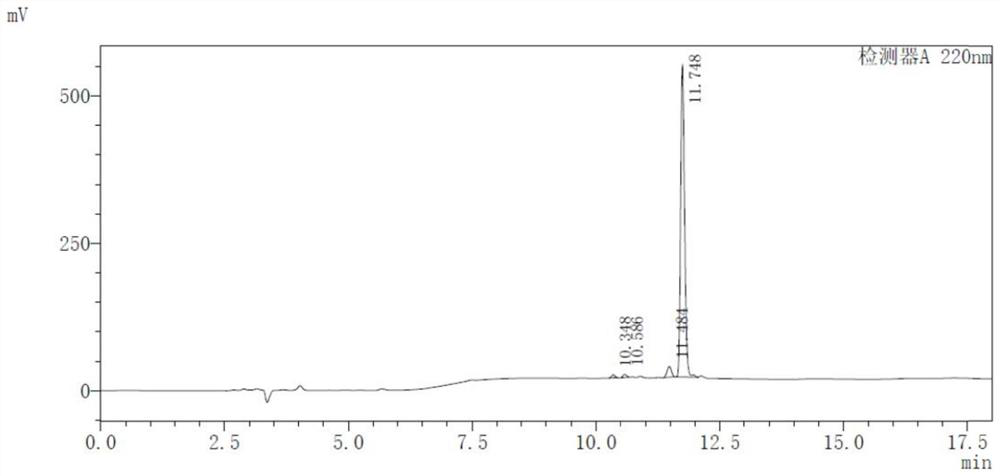
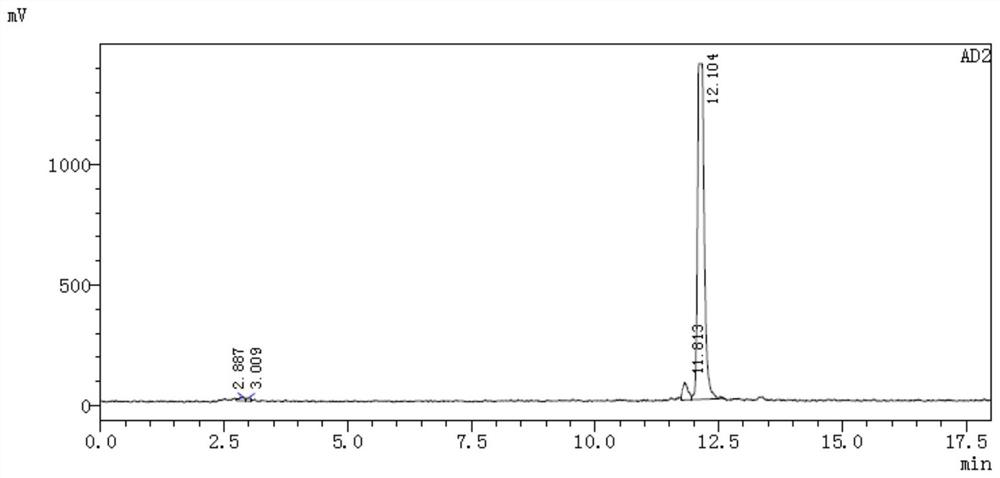
[0059] 68 The radiochemical stability of Ga-DOTA-ANCP-PSMA was carried out in two systems of calf serum and phosphate buffer. PBS method: placed in 0.5mL phosphate buffer solution (PBS, pH=7.4), placed at 37°C, incubated for 30, 60, 90, 120, and 150min, and then measured its radiochemical purity by HPLC to determine its in vitro stability. Serum method: place in 0.5mL calf serum solution, incubate at pH=7, 37°C. When incubated for 30, 60, 90, 120, and 150 min, the radiochemical purity was determined by HPLC to determine its in vitro stability.
[0060] according to Figure 4 The results showed that from 30 minutes to 150 minutes, the radiochemical purity decreased slightly, but the stability remained above 95%. 68 Ga-DOTA-ANCP-PSMA has good stability in PBS system. In the serum system, from 30 minutes onwards, the radiochemical purity decreased to a certain extent, and the radiochemical purity was lower than 95% in 1.5 hours. 68 The st...
Embodiment 3
[0062] Measurement of lipid-water partition coefficient:
[0063] Mark 68Ga-DOTA-ANCP-PSMA was separated and purified by Sep-Pak C18 Cartridge, and eluted with 95% ethanol. The obtained marker was blown dry by dry nitrogen, and the obtained marker was dissolved in a 1.5mL EP tube (about 3.7MBq) using the same volume (0.5mL: 0.5mL) of n-octanol and phosphate buffer solution (pH=7.4) middle. Fully shake for 5 minutes, and centrifuge the layers in a centrifuge for 5 minutes at a speed of 2000 rpm. Take 100uL each of the organic phase and the aqueous phase in 1mL EP tubes, measure their radioactive counts in the well-type γ detector, and calculate the lipid-water partition coefficient P from the ratio of the radioactive counts of the organic phase and the aqueous phase. P=log (N O / N W )(N O and N W are the counts of the organic and aqueous phase samples, respectively). The operation was repeated 3 times, and the average value was taken as the lipid-water partition coeffic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com