Ferrocenyl-containing thiadiazolyl Schiff base and preparation method thereof
A technology based on thiadiazolyl and ferrocene, which is applied in the field of Schiff base containing ferrocenyl thiadiazolyl and its preparation, can solve the problems of long reflux time, high reaction temperature, low yield and the like, and achieves the reaction The effect of short time, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
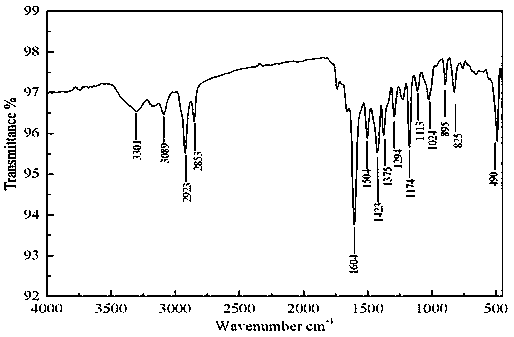
[0037] Example 1 Preparation of acetyl ferrocene 2-amino-1,3,4-thiadiazole Schiff base:
[0038] Add 1.4g (10mmol) of choline chloride in a dry three-necked flask, 1.92 (20mmol) of methanesulfonic acid, stir at room temperature to obtain a deep eutectic solvent, then add 0.23g (1mmol) acetyl ferrocene, 0.12 g (1.2 mmol) 2-amino-1,3,4-thiadiazole was reacted in a water bath at 40°C, and monitored by TLC until the reaction was complete. After the reaction, the reaction solution was poured into water, extracted with dichloromethane, and the solvent was distilled off to obtain a crude product, and the water phase was recovered to obtain a deep eutectic solvent again. The crude product was recrystallized from absolute ethanol to obtain the pure product containing ferrocenyl thiadiazolyl Schiff base. The yield is 94.2%, and the melting point is 148-149°C.
[0039] IR(KBr) ν: 3301cm -1 ,3089cm -1 ( ν 二茂铁C-H ); 2923cm -1 ( ν CH3 ); 1375cm -1 ( ν CH3 ) ;1604cm -1 ( ν...
Embodiment 2
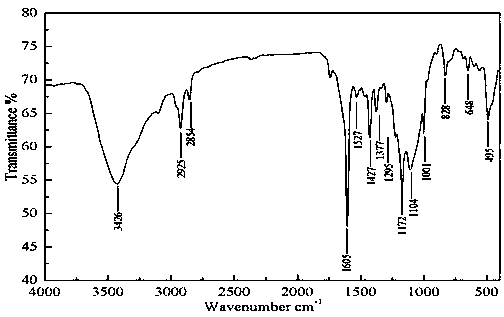
[0041] Example 2 Preparation of acetyl ferrocene 2-amino-5-methyl-1,3,4-thiadiazole Schiff base
[0042] Add 1.4g (10mmol) of choline chloride in a dry three-necked flask, 1.92 (20mmol) of methanesulfonic acid, stir at room temperature to obtain a deep eutectic solvent, then add 0.23g (1mmol) acetyl ferrocene, 0.14 g (1.2 mmol) 2-amino-5-methyl-1,3,4-thiadiazole was reacted in a water bath at 40°C, and monitored by TLC until the reaction was complete. After the reaction, the reaction solution was poured into water, extracted with dichloromethane, and the solvent was distilled off to obtain a crude product, and the water phase was recovered to obtain a deep eutectic solvent again. The crude product was recrystallized from absolute ethanol to obtain the pure product containing ferrocenyl thiadiazolyl Schiff base. The yield is 90.3%, and the melting point is 150-152°C.
[0043] IR(KBr) ν: 3426cm -1 ( ν 二茂铁C-H ); 2925cm -1 ( ν CH3 ); 1377cm -1 ( ν CH3 ) ; 1605cm ...
Embodiment 3
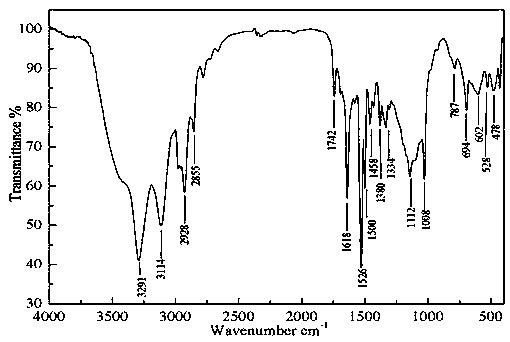
[0045] Example 3 Preparation of acetyl ferrocene 2-amino-5-ethyl-1,3,4-thiadiazole Schiff base
[0046] Add 1.4g (10mmol) of choline chloride in a dry three-necked flask, 1.92 (20mmol) of methanesulfonic acid, stir at room temperature to obtain a deep eutectic solvent, then add 0.23g (1mmol) acetyl ferrocene, 0.15 g (1.2 mmol) 2-amino-5-ethyl-1,3,4-thiadiazole was reacted in a water bath at 40°C, and monitored by TLC until the reaction was complete. After the reaction, the reaction solution was poured into water, extracted with dichloromethane, and the solvent was distilled off to obtain a crude product, and the water phase was recovered to obtain a deep eutectic solvent again. The crude product was recrystallized from absolute ethanol to obtain the pure product containing ferrocenyl thiadiazolyl Schiff base. The yield is 91.7%, and the melting point is 145-146°C.
[0047] IR(KBr) ν: 3291cm -1 ,3114cm -1 ( ν 二茂铁C-H ); 2928cm -1 ( ν CH3 ); 1742cm -1 , 1380cm -1 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com