Preparation method of high-purity lenvatinib and salt thereof
A technology of lenvatinib and compounds, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as increased process costs, achieve the effects of reducing post-processing steps, controlling residues, and reducing the production of impurities 10 and 11
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
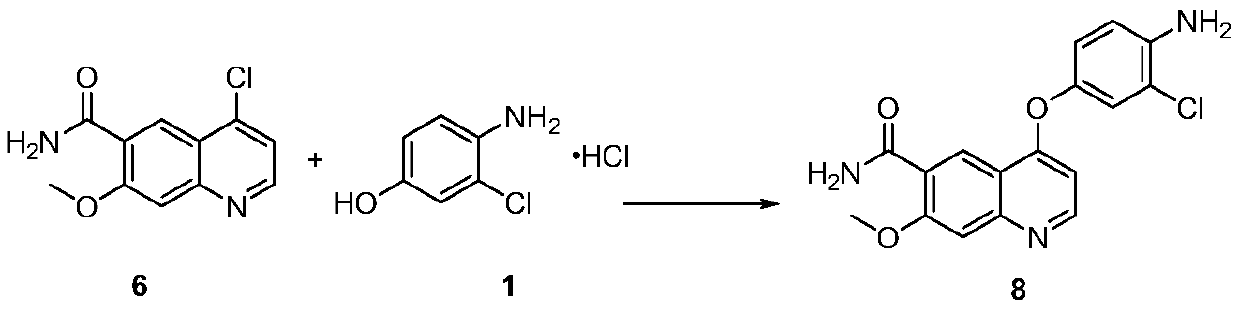
[0052] Example 1: Preparation of 4-(4-amino-3-chlorophenoxy)-7-methoxyquinoline-6-formamide
[0053]
[0054] Add 900ml of dimethyl sulfoxide and 90g of 3-chloro-4-aminophenol hydrochloride (Formula 1) into the reaction flask, add dropwise 120g of 48w / w% potassium hydroxide aqueous solution, after the dropwise addition is complete, stir at room temperature for 0.5h. Add 90 g of 4-chloro-7-methoxyquinoline-6-carboxamide (Formula 6). Heating reaction 14 ~ 15h. Add 1800ml of acetone aqueous solution (volume ratio 1:3) dropwise, stir for 1h after the completion of the dropwise addition, cool down to room temperature after stirring, crystallize for 2h, filter, wash with 1300ml of acetone aqueous solution (volume ratio 1:3), and dry. 4-(4-Amino-3-chlorophenoxy)-7-methoxyquinoline-6-carboxamide (Formula 8) was obtained.
[0055] Table 1 Effect of different reaction temperatures on yield and purity
[0056]
[0057] It can be seen from the results in Table 1 that when the tem...
Embodiment 2
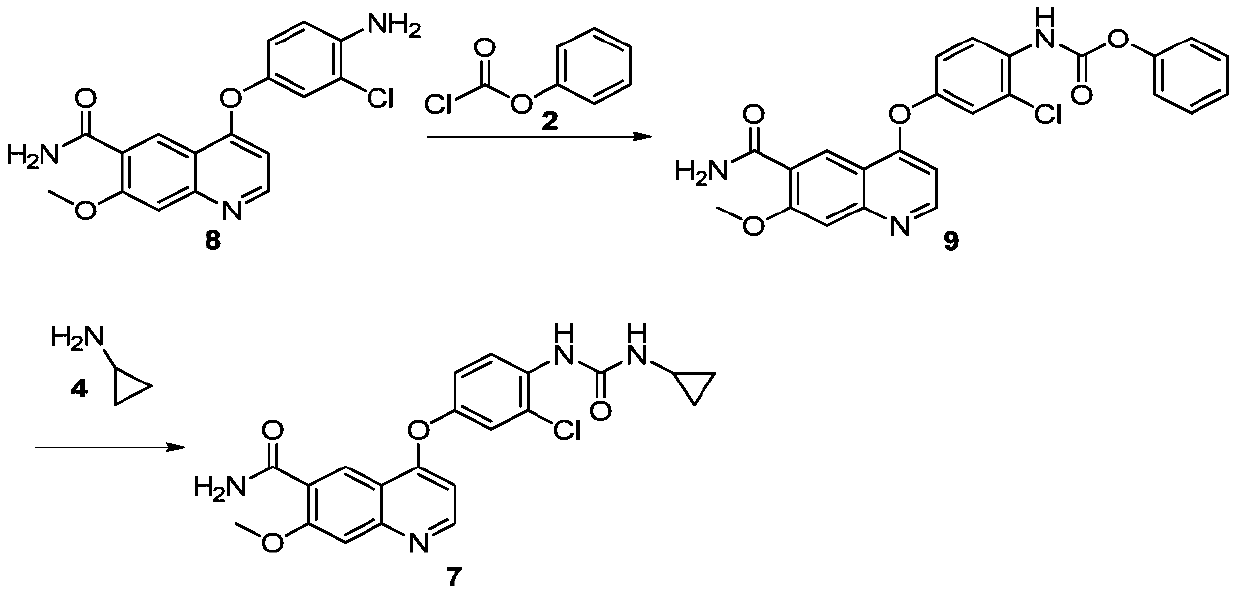
[0058] Embodiment 2: Preparation of {4-[(6-carbamoyl-7-methoxy-4-quinolyl)oxy]-2-chloroanilino}formic acid phenyl ester
[0059]
[0060] Add 50 g of 4-(4-amino-3-chlorophenoxy)-7-methoxyquinoline-6-carboxamide (formula 8) obtained in Experiment 1-2 of Example 1 into the reaction flask and add N,N - 400ml of dimethylformamide, 30g of pyridine and 3g of purified water, reduce to the reaction temperature, add 50g of phenyl chloroformate (Formula 2) dropwise, and react for 2h after the dropwise completion. 1000ml of acetone was added dropwise, stirred and crystallized. Filter, wash with 200ml of acetone, and dry to obtain phenyl {4-[(6-carbamoyl-7-methoxy-4-quinolyl)oxy]-2-chloroanilino}formate (Formula 9).
[0061] Under the condition that the above conditions remain unchanged, only the conditions shown below are changed to affect the compound of formula 9.
[0062] Table 2 Effect of different reaction temperatures
[0063]
[0064] In the prior art, the temperature is ...
Embodiment 3
[0065] Embodiment 3: the preparation of lenvatinib
[0066]
[0067] Add 80g of the compound of formula 9 obtained in Experiment 2-2 in Example 2 and 560ml of N,N-dimethylformamide into the reaction flask, add 34.5g of cyclopropylamine dropwise at 13°C, and keep the temperature for 2h. Add 1260ml of acetone aqueous solution (volume ratio 20:1) for crystallization, and stir for 2 hours after dropping. After filtering, washing with 160ml of acetone, and drying, 54g of delenvatinib was obtained, and the yield was 73%. Calculate the amount of lenvatinib content and impurities, and the results are shown in Table 3.
[0068] Table 3 Contents of different impurities in lenvatinib
[0069]
[0070] Note: "RT" is the retention time.
[0071] According to the threshold of toxicological concern stipulated in the "Guidelines on the Limits of Genotoxic Impurities" issued by the European Medicines Agency, the content of the compounds of formula 6 and formula 11 is 60ppm (0.006%) or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com