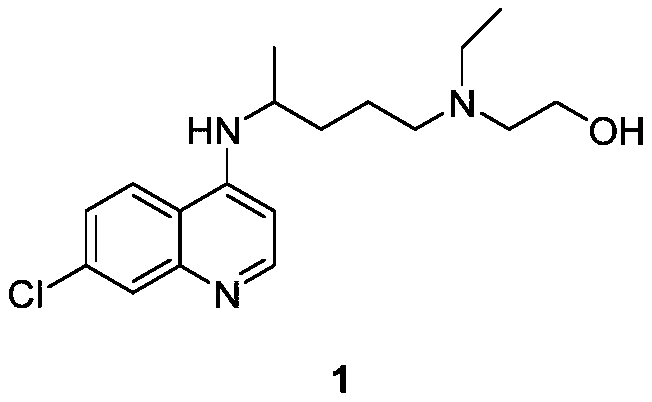
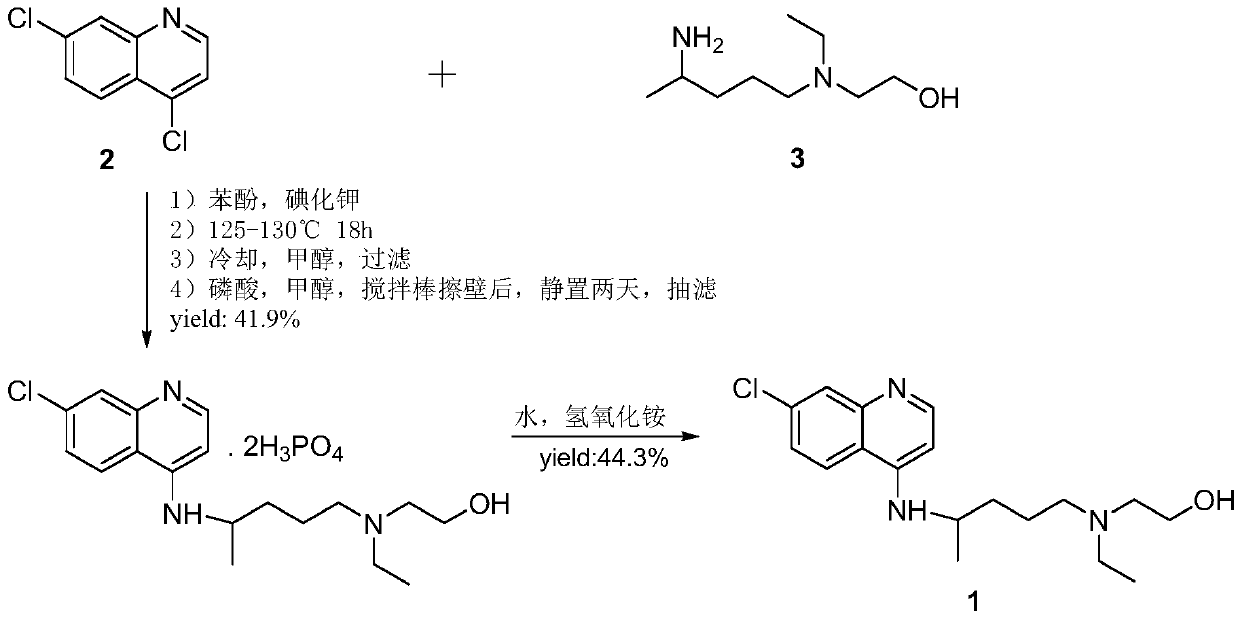
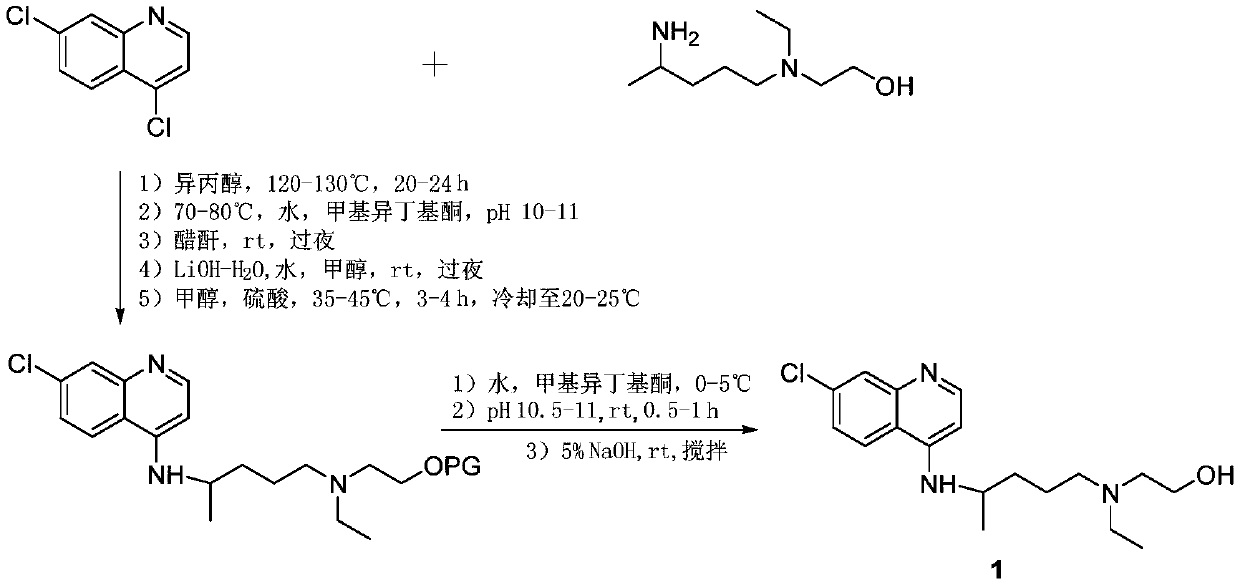
Hydroxychloroquine synthetic method
A synthetic method, the technology of hydroxychloroquine, which is applied in the field of preparation of hydroxychloroquine, can solve the problems of expensive raw materials, difficult recovery of solvents, and unfriendly environment, and achieve the effects of enhancing market competitiveness, mild reaction conditions, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0048] Add 4,7-dichloroquinoline (500g, 2.5mol), 5-(N-ethyl-N-2-hydroxyethylamino)-2-pentylamine ( 668g, 3.8mol) and N,N-diisopropylethylamine (323g, 2.5mol), pass through nitrogen protection, start mechanical stirring, slowly raise the temperature to 125~135°C for 8h under reflux; cool the reaction solution, and wait to concentrate The liquid was cooled to below 50°C, and 1500mL of water was added; after stirring for 15min, the reaction liquid dropped below 40°C, extracted with isopropyl acetate (3000mL*3), and the organic phase was washed with water (3000mL*2), and washed with saturated brine ( 3000mL*1), 2 / 3 isopropyl acetate was recovered under reduced pressure, and then slowly lowered to 0-5°C for 1 hour; suction filtration with a Buchner funnel yielded 801g (wet weight) of an off-white solid, which was then weighed by isopropyl acetate The wet weight of a white powdery solid was 710 g after crystallization, and it was vacuum-dried at 40° C. for 6 hours to obtain 627 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com