Preparation method of metal-layered double hydroxide composite electrode material
A layered bimetallic and hydroxide technology, applied in the direction of electrodes, electrolytic processes, electrolytic components, etc., to achieve multi-functional excellence, promote catalysis, and promote the effect of electrolytic water process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of iron-nickel alloy-iron-nickel layered double hydroxide
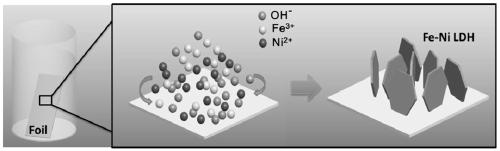
[0031] see figure 1 In the process shown, the iron-nickel alloy sheet (20×40×0.3mm) was cleaned with acetic acid (2M) to remove the surface oxide layer, and then ultrasonically cleaned in ethanol and deionized water for ten minutes. Dissolve nickel nitrate hexahydrate (0.5 mmol), ferric nitrate nonahydrate (0.5 mmol) and urea (5 mmol) in 36 ml of deionized water, and stir until completely dissolved. The solution was then transferred to a steel autoclave, and the prepared iron-nickel alloy foil was then completely immersed in the reaction liquid. The sealed polytetrafluoroethylene-lined stainless steel autoclave (50ml) was transferred to an oven to react at 120°C for 12h, and then naturally cooled to room temperature. Then the reacted iron-nickel alloy foil was taken out, rinsed several times with ethanol and deionized water, and finally placed in a vacuum oven at 80° C. for 6 hours and then ta...
Embodiment 2
[0035] (1) Preparation of iron-iron-nickel layered double hydroxide
[0036] Preparation of iron-iron-nickel layered double hydroxide: except that the iron-nickel alloy foil is replaced by iron foil, the rest is the same as in Example 1.
[0037] (2) Iron-iron-nickel layered double hydroxide for oxygen evolution reaction in water electrolysis
[0038] Iron-iron-nickel layered double hydroxide is used for electrolytic water oxygen evolution reaction: same as embodiment 1, but the reaction efficiency is different, such as Figure 4 shown. Iron-iron-nickel layered double hydroxide, in 0.1M KOH electrolyte, the oxygen evolution reaction has a start-up overpotential of 210mV, and at a current of 10mA / cm 2 The reaction overpotential was 390mV. .
Embodiment 3
[0040] (1) Preparation of nickel-iron-nickel layered double hydroxide
[0041] Preparation of nickel-iron-nickel layered double hydroxide: same as in Example 1, only need to replace the iron-nickel alloy foil with nickel foil.
[0042] (2) Nickel-iron-nickel layered double hydroxide for electrolysis of water oxygen evolution reaction
[0043] Nickel-iron-nickel layered double hydroxide is used for electrolytic water oxygen evolution reaction: same as embodiment 1, but the reaction efficiency is different, such as Figure 4 shown. Iron-iron-nickel layered double hydroxide, in 0.1M KOH electrolyte, the oxygen evolution reaction has a start-up overpotential of 220mV, and at a current of 10mA / cm 2 The reaction overpotential was 410mV. .
[0044] figure 2 and image 3 Be respectively the scanning electron microscope picture and the transmission electron microscope picture of the composite electrode material of the different metal bases that are obtained in embodiment 1 to em...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com