A small molecule heterocyclic dimer and use
A dimer and small molecule technology, which is applied in the application field of small molecule heterocyclic dimer and the preparation of antitumor drugs, can solve the problems of chemotherapy effectiveness, drug toxicity and drug resistance, and achieve good therapeutic effect. , the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0089] Example 2 Synthesis of Decanediamine-Ligustrazine Dimer
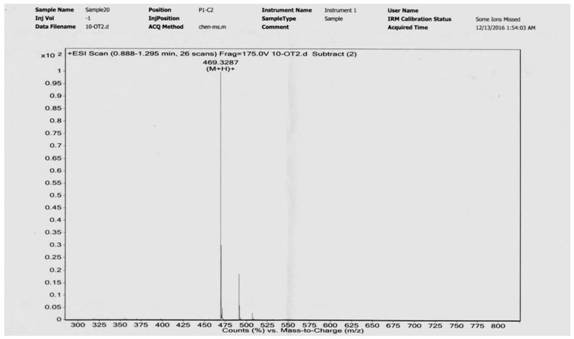
[0090] Add 0.530g ligustrazine acid, 0.723g EDCI, 1.33mL triethylamine, 10mL anhydrous dichloromethane to the round-bottomed flask, stir to dissolve, then add 0.250g decanediamine, stir and react at room temperature for 12h, add 20mL dichloromethane , washed successively with water (2×30 mL) and saturated brine (1×30 mL), dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure. The residue was separated by silica gel column chromatography, and the eluent (petroleum ether / acetone=5 / 1-4 / 1) was used to obtain 0.451 g of a white solid with a yield of 66% (see the synthetic route diagram). image 3 , see the characterization map Figure 4 ).
Embodiment 3 12
[0091] Example 3 Synthesis of dodecanediamine-ligustrazine dimer
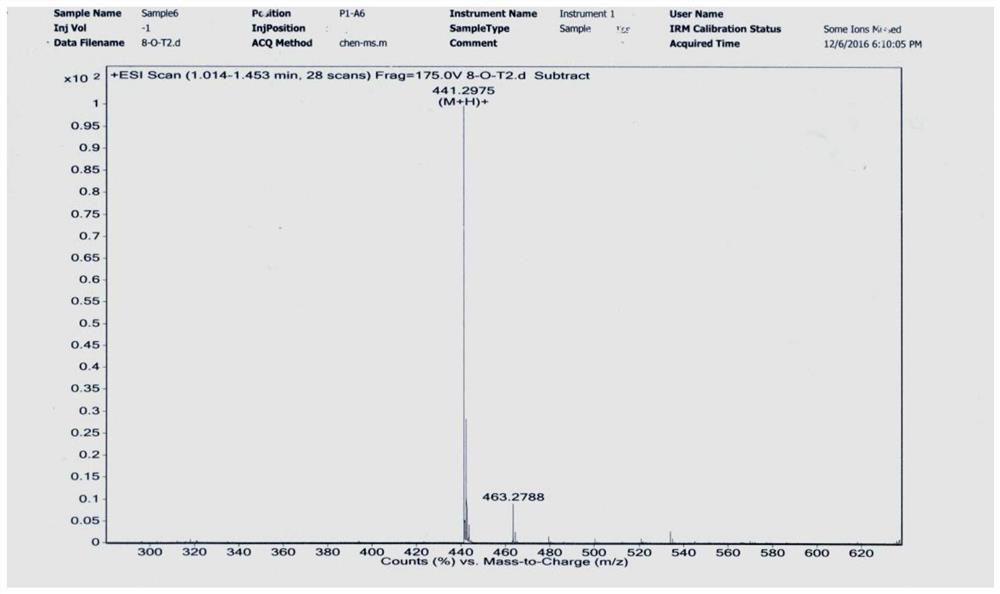
[0092] Add 0.456g ligustrazine acid, 0.621g EDCI, 1.14mL triethylamine, 10mL anhydrous dichloromethane into the round-bottomed flask, stir to dissolve, then add 0.250g dodecanediamine, stir and react at room temperature for 12h, add 20mL dichloromethane Chloromethane was washed successively with water (2×30 mL) and saturated brine (1×30 mL), dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure. The residue was separated by silica gel column chromatography, and the eluent (petroleum ether / acetone=5 / 1-4 / 1) was used to obtain 0.445 g of white solid with a yield of 72% (see the synthetic route diagram). Figure 5 , see the characterization map Image 6 ).
Embodiment 4
[0093] Example 4 Synthesis of decanediamine-cinnamic acid dimer
[0094] 0.400 g of cinnamic acid, 0.211 g of decanediamine, 0.517 g of EDCI, 0.030 g of DMAP, and 10 mL of anhydrous dichloromethane were sequentially added to the round-bottomed flask, and the reaction was stirred at room temperature for 12 h. A white solid was precipitated, which was filtered with suction, washed with dichloromethane (2×2.5 mL) and water (3×5 mL) in turn, and dried to obtain 0.329 g of a white solid with a yield of 62% (see the synthetic route diagram). Figure 7 , see the characterization map Figure 8 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com