Methods of treating cancer
A cancer and cancer cell technology, applied in biochemical equipment and methods, pharmaceutical formulations, microbial measurement/inspection, etc., can solve the problem of reducing the proliferation ability of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
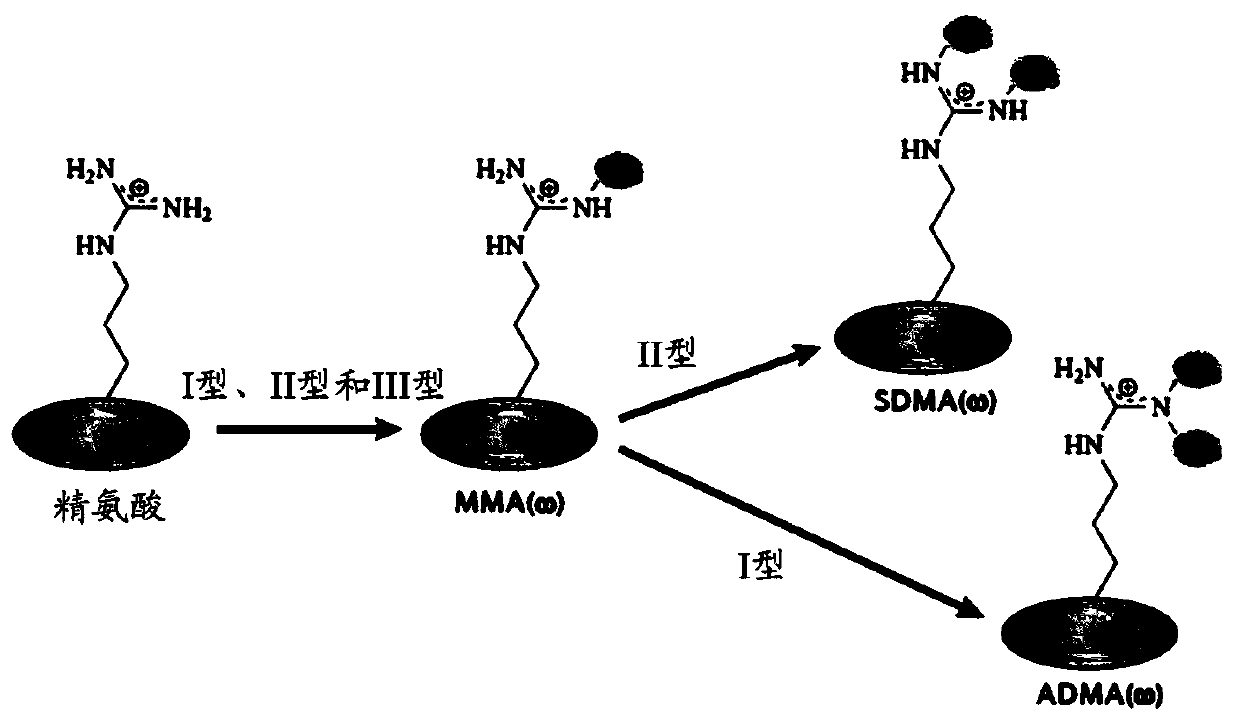
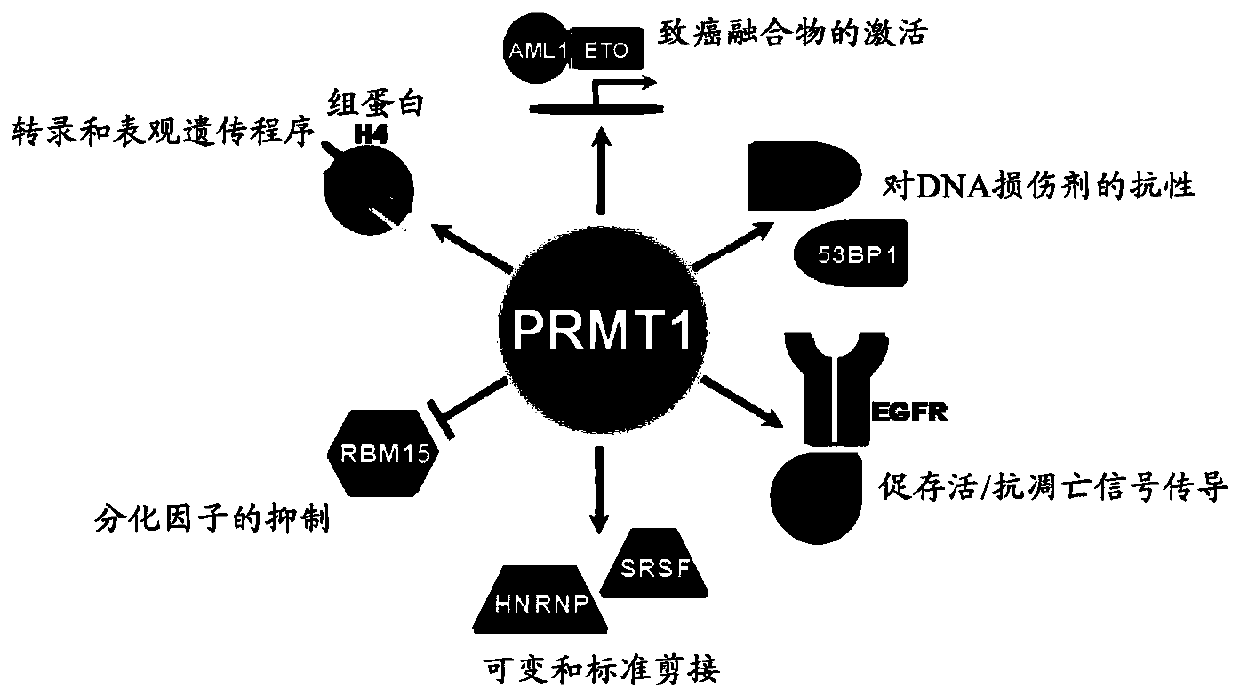
[0283] Arginine methylation and PRMT
[0284] Arginine methylation is an important post-translational modification of proteins involved in various cellular processes, such as gene regulation, RNA processing, DNA damage response, and signal transduction. Proteins containing methylated arginine are present in the nucleus and cytoplasmic components, indicating that enzymes that catalyze the transfer of methyl groups to arginine are also present in these subcellular compartments (reviewed in Yang, Y. & Bedford, MTProtein arginine methyltransferases and cancer.Nat Rev Cancer13,37-50,doi:10.1038 / nrc3409(2013); Lee,YH&Stallcup,MRMinireview:proteinarginine methylation of nonhistone proteins in transcriptional regulation.MolEndocrinol23,425-433,doi:10.1210 / me.2008-0380(2009)). In mammalian cells, methylated arginine exists in three main forms: ω-N G -Monomethyl-arginine (MMA), ω-N G ,N G -Asymmetric dimethylarginine (ADMA) or ω-N G ,N’ G -Symmetric dimethylarginine (SDMA). Each methyla...
Embodiment 2
[0368] Predictive biomarkers
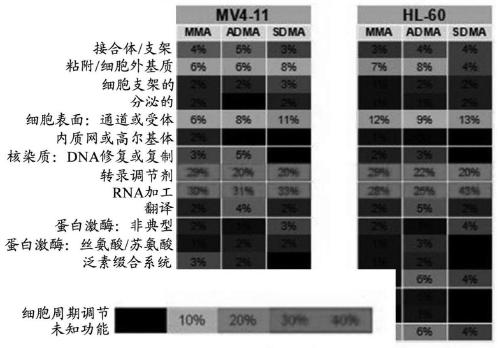
[0369] The sensitivity ranking of the cell line to compound A (by gIC50) and the relationship with somatic changes or gene expression were examined using genomic data obtained through Cancer Cell Line Encylopedia (CCLE). In addition, lymphoma cell lines are graded by their ability to subject Compound A to a cytotoxic response. Using this method, it can be determined that there is no obvious correlation with any cancer-related changes, which may be due to the extensive activity of compound A in cell culture. Therefore, a reasonable approach was investigated based on the combined activity observed with PRMT5 inhibition.
[0370] Recent studies describe the mechanism by which the loss of 5-methylthioadenosine phosphorylase (MTAP) gene may inhibit endogenous PRMT5 in tumor cells. The MTAP gene is frequently deleted in cancers, including 40% of glioblastoma, 25% of melanoma and pancreatic cancer, and 15% of non-small cell lung cancer. (Mavrakis, KJ et a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com