Drug-modified intraocular lens and preparation method and application thereof
An intraocular lens and drug technology, which is used in drug combinations, pharmaceutical formulations, prostheses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of the intraocular lens of embodiment 1 drug modification
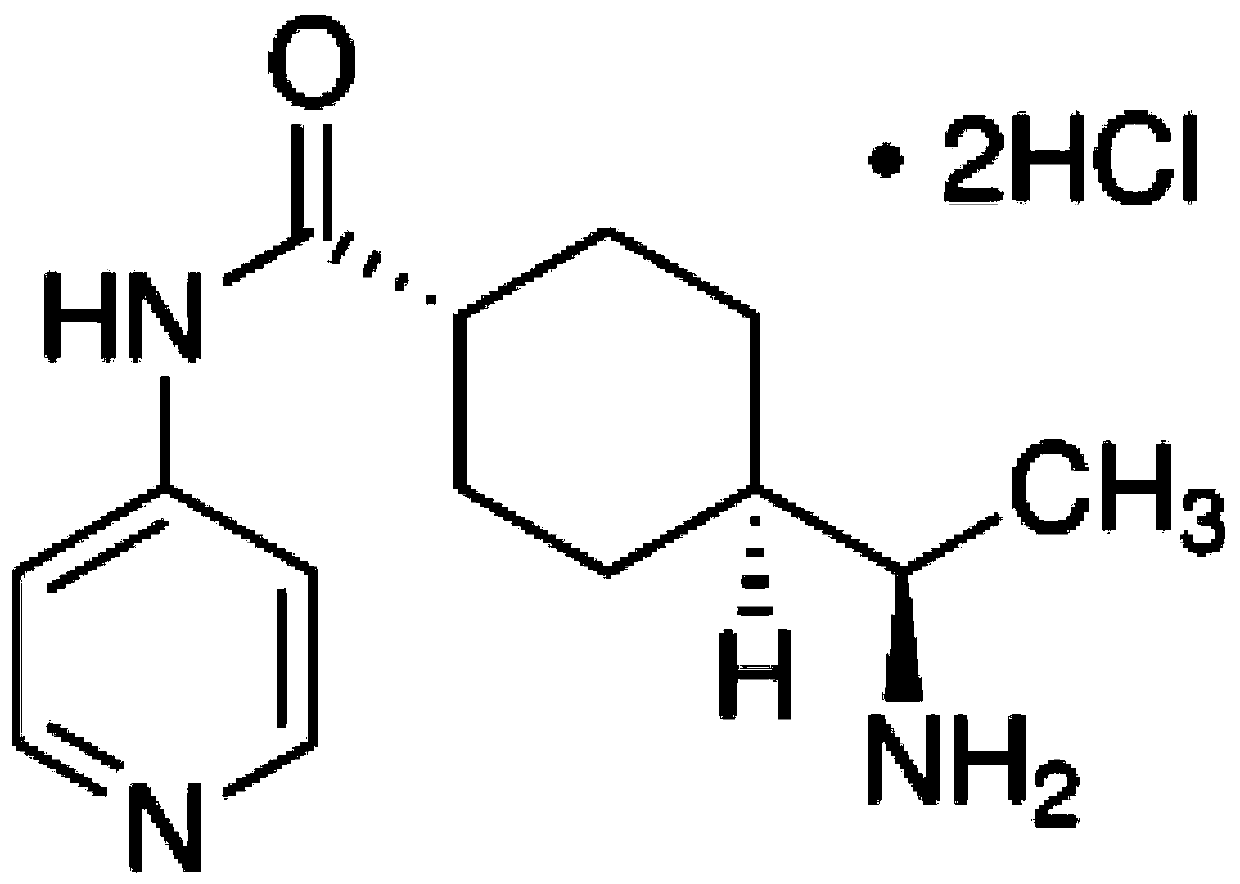
[0043] The medicine used in the present invention is Y27632, empirical molecular formula C 14 h 21 N 3O.2HCl, molecular weight: 320.26. Molecular structure such as figure 1 shown.
[0044] The modification material used in the present invention is polylactic acid-glycolic acid copolymer, linear molecular formula [C 3 h 4 o 2 ] x [C 2 h 2 o 2 ] y , lactic acid: glycolic acid 50:50, molecular weight: 24,000-38,000, molecular structure formula such as figure 2 shown.
[0045] 1. Preparation of main reagents
[0046] (1) Y27632 solution: 1 mg of Y27632 powder was added to 78.04 μl of sterilized double distilled water to prepare a concentration of 40 mM, and stored at 4°C.
[0047] (2) 1% PLGA solution: 1 mg of PLGA powder was dissolved in 0.1 ml of chloroform, and stored at -20°C.
[0048] 2. Intraocular lens simulation material modification
[0049] Take out the intraocular lens, ...
Embodiment 2
[0052] The drug effect of the intraocular lens modified by the drug of embodiment 2
[0053] 1.1 In vitro release test
[0054] Take sterile 1.5ml centrifuge tubes, add 1.0ml of sterilized PBS to each tube, place the drug-modified IOL in PBS, and shake on a constant temperature shaker at 37°C at a frequency of 100rpm for 15min, 30min, 1h, At 2h, 4h, 8h, 12h, 24h, and 48h, take out 200ul of the solution to a sterile centrifuge tube, then add 200ul of sterilized PBS, and put it back on the shaker. After 48 hours of testing, all samples were collected for testing. After zeroing the UV spectrophotometer equipment with PBS, carry out absorbance scanning at 200-800nm wavelength on the solution of Y27632 gradient dilution (40μM, 20μM, 10μM, 5μM, 2.5μM, 1.35μM, 0.625μM, 0.3125μM, 0.15625μM) , used to make the standard curve of drug standards, the measurement sequence is from low concentration to high concentration. Then carry out the absorbance scanning of 200-800nm wavelength ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com