Benzodithiophene and pyrazine copolymer, preparing method and application
A benzodithiophene, pyrazine copolymer technology, applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components and other directions, can solve problems such as limiting photoelectric conversion efficiency, achieve wide absorption spectrum, good photoelectric conversion characteristics, good The effect of photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
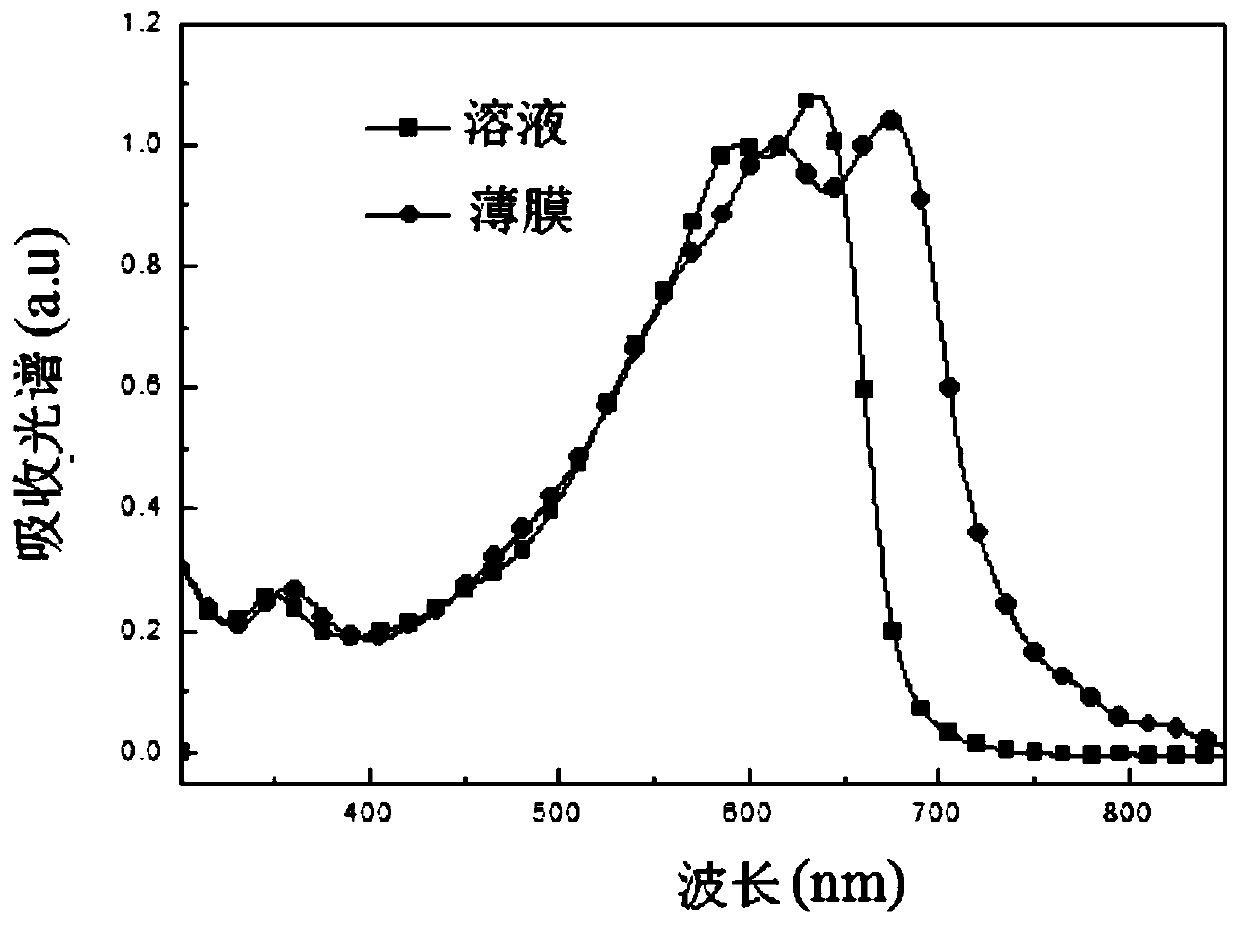
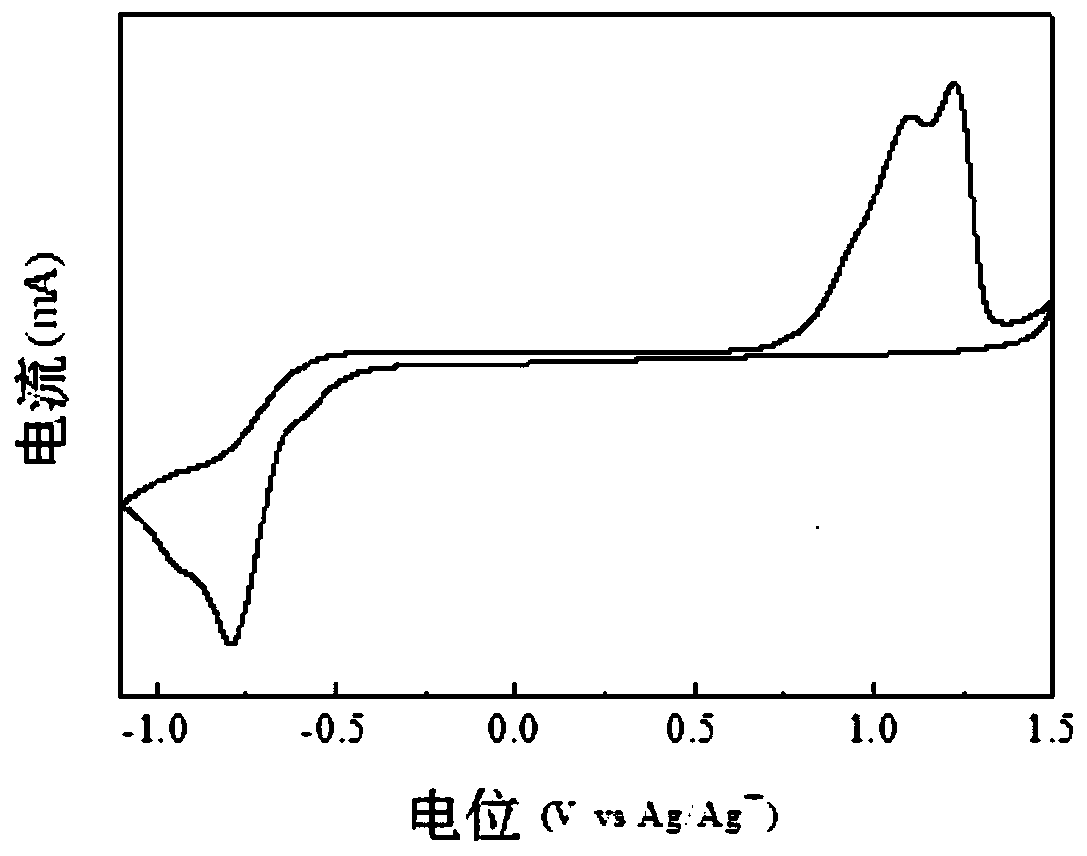
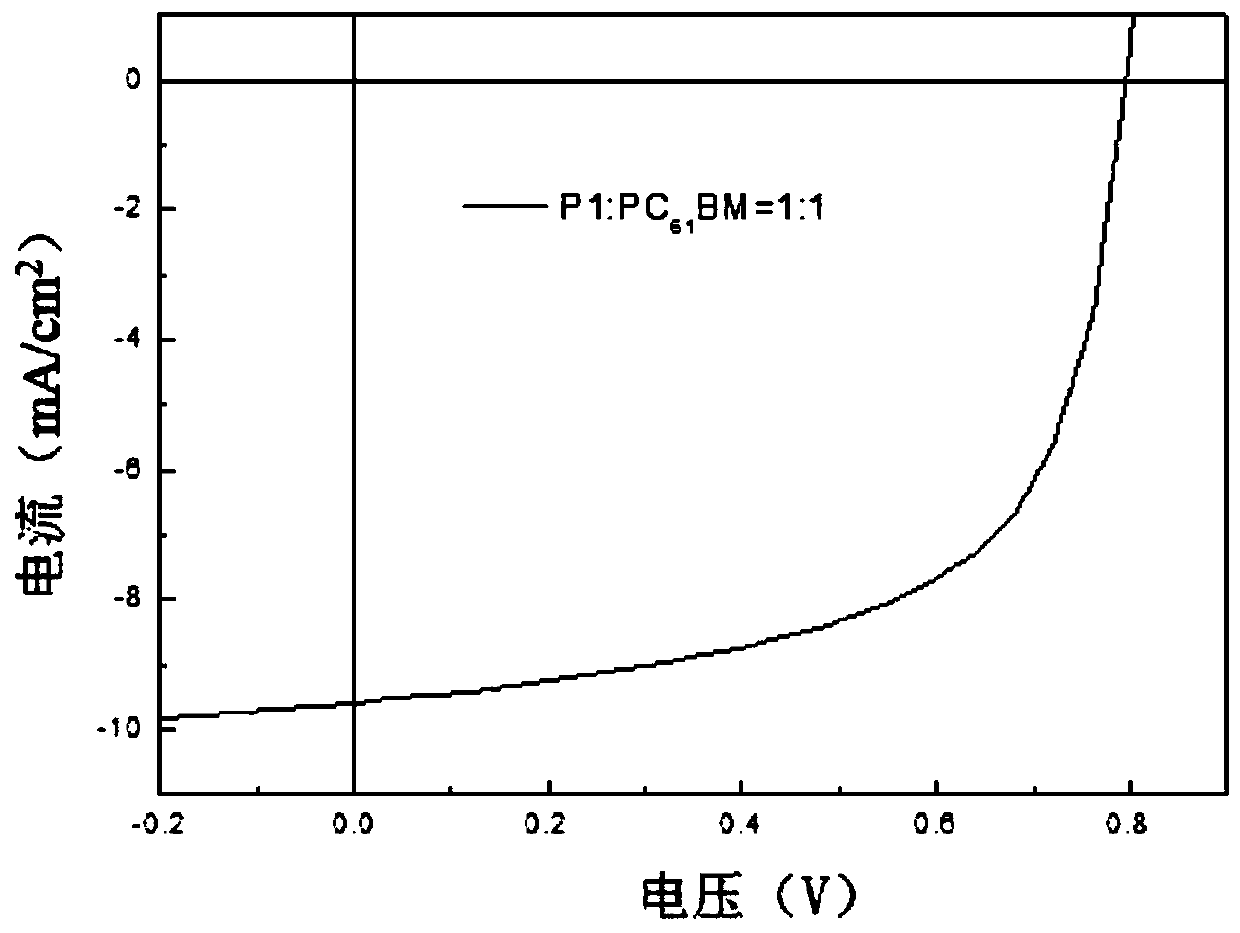
Image
Examples
Embodiment 1
[0053] 4,8-Diisooctyloxy-benzo(1,2-b; 4,5-b′)-dithiophene-2,5-diisooctyloxy-3,6-bis(furan-2- Preparation of methylene)pyrazines (P1).
[0054] ①Synthesis of Compound 1
[0055] 1,4-Diacetylpiperazine-2,5-dione (1.19g, 6mmol) and 5-bromo-2 furfural (2.63g, 15mmol) were placed in a 50ml single-necked round bottom flask, and then 28ml DMF, under the protection of nitrogen, heat and stir, raise the temperature to 120°C, add 3.32ml of triethylamine through a syringe, after the completion, the reaction system turns from colorless to red immediately, continue to react for 12 hours, after the completion of the reaction, cool to room temperature, produce The yellow precipitate was collected by filtration, washed with acetone, and the product was collected to obtain the target product (2.16 g, yield: 84%), which was directly carried out to the next reaction without further purification.
[0056] The nuclear magnetic resonance spectrum and mass spectrum of compound 1 are as follows:
...
Embodiment 2
[0069] Example 2 4,8-Diisooctyloxy-benzo(1,2-b; 4,5-b')-dithiophene-2,5-dioctyloxy-3,6-bis(furan- Preparation of 2-methylene)pyrazine (P2)
[0070] The synthetic method of compound 2 is the same as the synthetic method of compound 2 in embodiment 1, and brominated alkane adopts brominated n-octane, and concrete steps are as follows:
[0071] ①Synthesis of Compound 1
[0072] 1,4-Diacetylpiperazine-2,5-dione (1.19g, 6mmol) and 5-bromo-2 furfural (2.63g, 15mmol) were placed in a 50ml single-necked round bottom flask, and then 28ml DMF, under the protection of nitrogen, heat and stir, raise the temperature to 120°C, add 3.32ml of triethylamine through a syringe, after completion, the reaction system turns from colorless to red immediately, continue to react for 12h, after the reaction is completed, cool to room temperature, and produce yellow The precipitate was collected by filtration, washed with acetone, and the product was collected to obtain the target product (2.16 g, yie...
Embodiment 3
[0086] Example 3 4,8-Diisooctyloxy-benzo(1,2-b; 4,5-b')-dithiophene-2,5-bis(2-octyldodecyloxy)- Preparation of 3,6-bis(furan-2-methylene)pyrazine (P3)
[0087] The synthetic method of compound 2 is the same as the synthetic method of compound 2 in embodiment 1, and brominated alkane adopts brominated 2-octyl-dodecane, and concrete steps are as follows:
[0088] ①Synthesis of Compound 1
[0089]1,4-Diacetylpiperazine-2,5-dione (1.19g, 6mmol) and 5-bromo-2 furfural (2.63g, 15mmol) were placed in a 50ml single-necked round bottom flask, and then 28ml DMF, under the protection of nitrogen, heat and stir, raise the temperature to 120°C, add 3.32ml of triethylamine through a syringe, after completion, the reaction system turns from colorless to red immediately, continue to react for 12h, after the reaction is completed, cool to room temperature, and produce yellow The precipitate was collected by filtration, washed with acetone, and the product was collected to obtain the target p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com