High-dispersion loaded noble metal catalytic material and preparation method thereof
A technology of catalytic materials and precious metals, which is applied in the field of highly dispersed and loaded noble metal catalytic materials and its preparation, can solve the problems of particle agglomeration, reduction of active component size, large size, etc., and achieve mild reaction process conditions, good conversion rate and selectivity , Ease of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
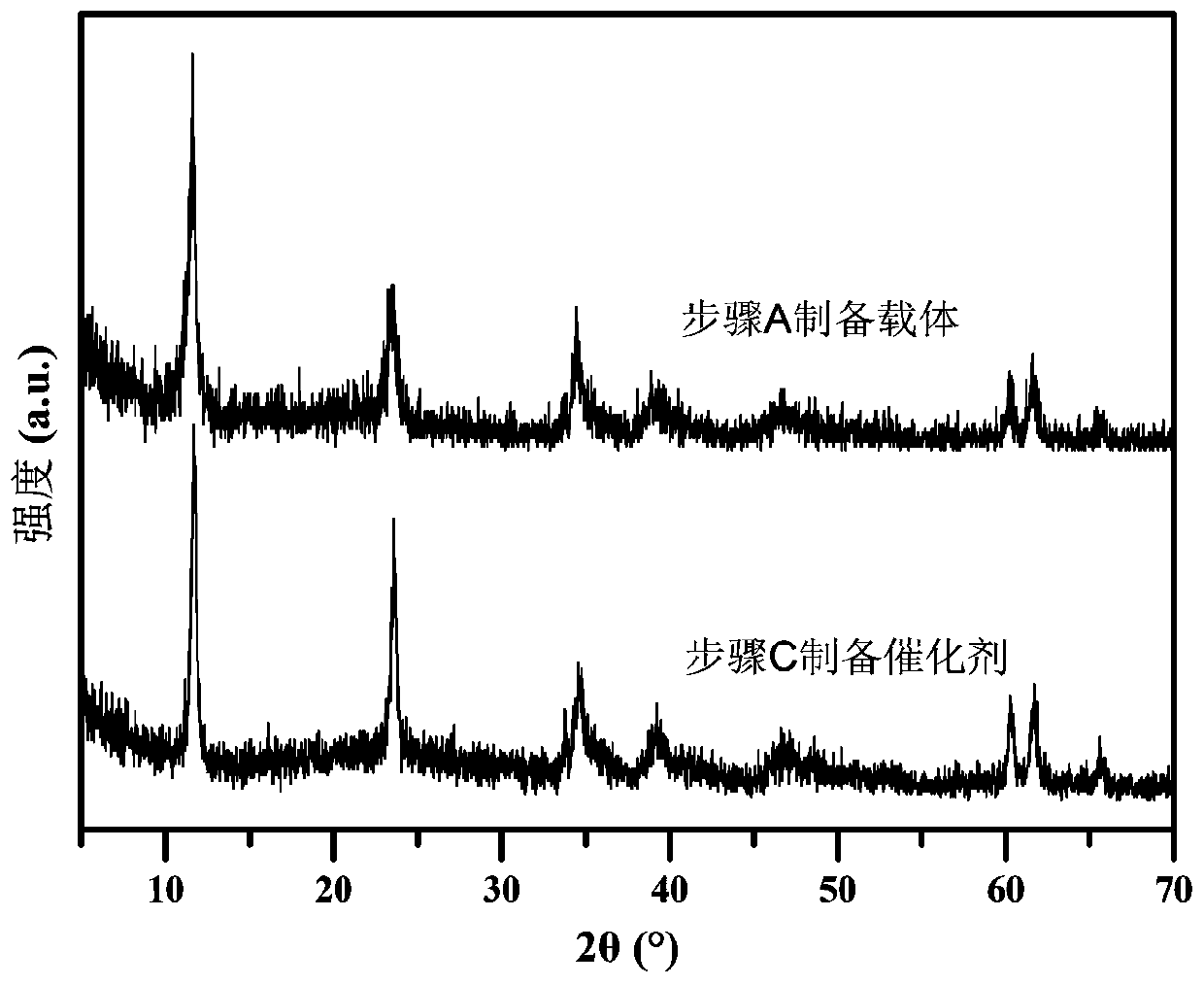
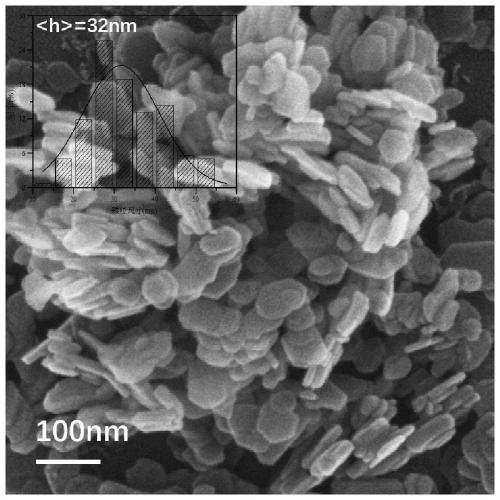
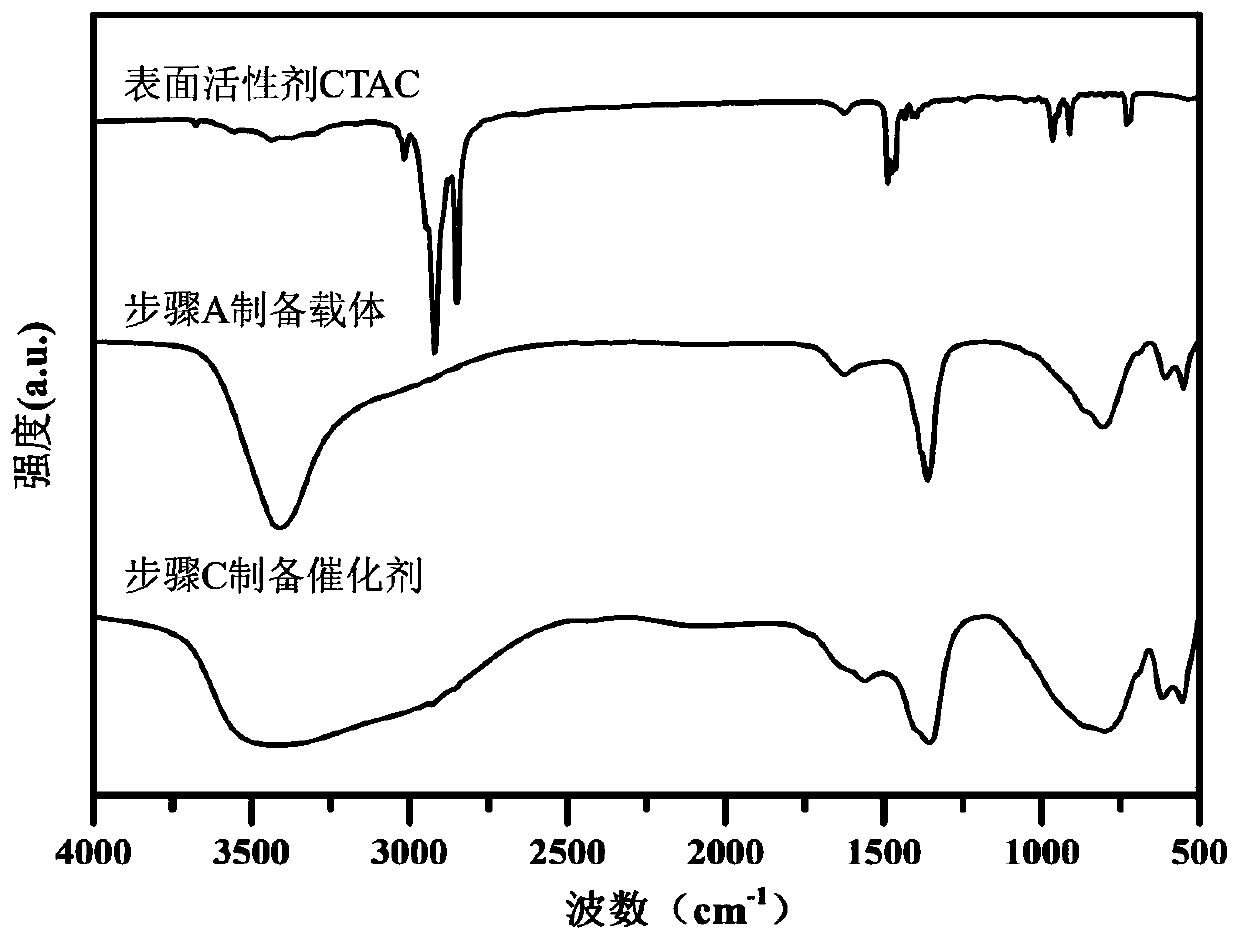
[0044] A. FeSO 4 ·7H 2 O 1.3901g, Al 2 (SO 4 ) 3 18H 2 O 1.6650g was dissolved in 150mL deionized water to prepare mixed salt solution; NaOH 0.6400g and NaOH 2 CO 3 1. Dissolve 0600g in 150mL deionized water to make a mixed alkali solution. The two were dropped into the flask at a constant speed, the volume ratio of the two was controlled to be 10:13, the pH was adjusted to 8.5-9.5, and the flask was kept tightly closed, and the reaction was stirred at a constant temperature of 60°C for 4 hours. Naturally cool to room temperature, filter, wash the precipitate until neutral, and dry at 60°C for 8 hours to obtain the reduced hydrotalcite carrier FeAl-LDH.
[0045] B. Weigh 0.5000 g of FeAl-LDH powder in step A and disperse it in 100 mL of surfactant CTAC solution with a concentration of 6.25 mmol / L, and stir at room temperature for 10-20 min to form a uniformly dispersed suspension;
[0046] C. Stir the suspension obtained in step B at a constant temperature at 0°C, and...
Embodiment 2
[0048] A. Mn(NO 3 ) 2 4H 2 O 1.6065g, Al(NO 3 ) 3 9H 2 O 1.2006g was dissolved in 150mL deionized water to prepare salt solution; NaOH 0.6144g and NaOH 2 CO 3 Dissolve 0.6783g in 150mL deionized water to prepare a mixed alkali solution. Drop the two into the flask at the same time and at a constant speed by the double drop method, control the volume ratio of the two to 5:7, adjust the pH to 8-9, stir and react at a constant temperature of 80°C for 10 hours, cool to room temperature naturally, filter and wash the precipitate To neutrality, dry at 60°C for 8 hours to obtain the reducing hydrotalcite carrier MnAl-LDH.
[0049] B. Weigh 0.5000 g of the MnAl-LDH powder in step A and disperse it in 100 mL of surfactant CTAB solution with a concentration of 10.85 mmol / L, and stir at room temperature for 15 min to form a uniformly dispersed suspension;
[0050] C. Stir the suspension obtained in step B at a constant temperature at 25°C, and at the same time add 785 microliter...
Embodiment 3
[0052] A. will Co(NO 3 ) 2 ·6H 2 O 2.9105g, Al(NO 3 ) 3 9H 2 O 1.8757g was dissolved in 150mL deionized water to prepare mixed salt solution; NaOH 0.9600g and NaOH 2 CO 3 1. Dissolve 0600g in 150mL deionized water to make a mixed alkali solution. The two were dropped into the flask at the same time at a constant speed by the double drop method, the volume ratio of the two was controlled to be 1:1, and the reaction was stirred at a constant temperature of 90°C for 2h. Cool naturally to room temperature, filter and wash the precipitate to neutrality, and dry at 60°C for 10 h to obtain CoAl-LDH, a reduced hydrotalcite carrier.
[0053] B. Weigh 0.5000 g of the CoAl-LDH powder in step A and disperse it in 100 mL of CTAC solution with a concentration of 6.25 mmol / L, and stir at room temperature for 15 minutes to obtain a uniformly dispersed suspension;
[0054] C. Stir the suspension obtained in step B at a constant temperature at a temperature of 50°C, and add 470 microli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com