Method for preparing deuterated five-membered aromatic heterocyclic compound under catalysis of silver
A technology for aromatic heterocycles and compounds, applied in the field of chemical synthesis, can solve the problems of inability to apply the final stage of drug development, poor functional group compatibility, and high production costs, and achieve the effects of being suitable for large-scale production, high compatibility, and stable deuteration rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: deuterated saconazole
[0021] Silver reagent (0.3 mmol), phosphine ligand (0.3 mmol), deuterated water (0.4 g, 20 mmol), saconazole (0.39 g, 1 mmol) and dimethyl sulfoxide ( 5 mL). 40 o After C reacted for 12 hours, a saturated ammonium chloride solution was added to quench the reaction, the mixed solution was extracted with dichloromethane, the organic phase was combined, the organic solvent was concentrated, and the product was further purified by column chromatography to obtain deuterated saconazole with a yield of 85%. . The deuterium substitution rate is 91%. 1 H NMR: δ 7.50 (s, 1 H), 7.46 (d, J = 2.4Hz, 1 H), 7.36 (d, J = 3.2 Hz, 1 H), 7.32 (d, J = 2.4 Hz, 1 H) , 7.08 (d, J =4.8 Hz, 0.09 H), 7.05 (s, 1 H), 6.91 (s, 1 H), 6.78 (s, 1 H), 4.97 (d, J =6.4 Hz, 1 H) , 4.53 (d, J = 9.6 Hz, 1 H), 4.28 (d, J = 9.6 Hz, 1 H), 4.20 (d, J = 12.0 Hz, 1 H), 4.04 (d, J = 12.0 Hz, 1 H); 13 C NMR: 157.85, 126.97, 118.92, 93.06, 61.72.
Embodiment 2
[0022] Embodiment 2: deuterated ticlopidine
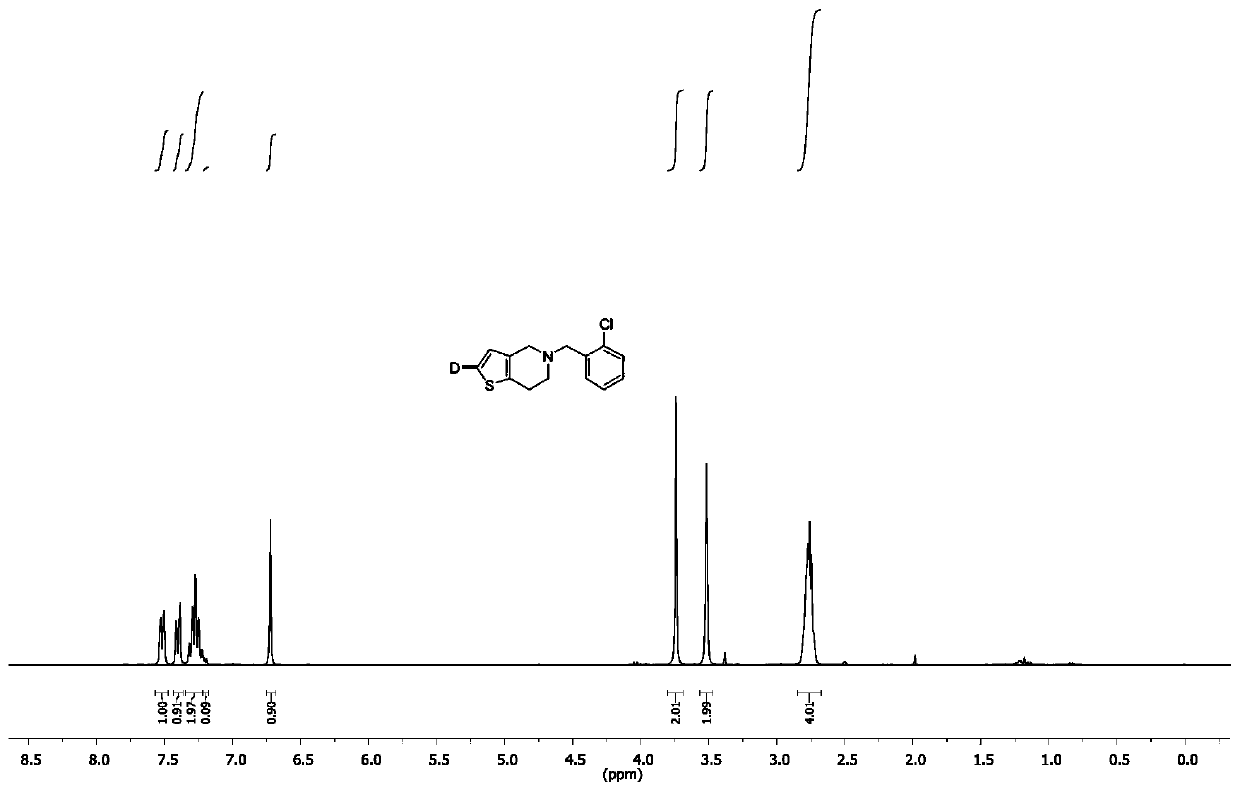
[0023] Add silver reagent (0.3 mmol), phosphine ligand (0.3 mmol), deuterated water (0.4 g, 20 mmol), ticlopidine (0.26 g, 1 mmol) and dimethyl sulfoxide into a 10 mL three-necked flask in sequence (5 mL). 40 o After C reacted for 12 hours, a saturated ammonium chloride solution was added to quench the reaction, the mixed solution was extracted with dichloromethane, the organic phase was combined, the organic solvent was concentrated, and the product was further purified by column chromatography to obtain deuterated ticlopidine with a yield of 95 %. The deuterium substitution rate is 91%. 1 H NMR: δ 7.52 (d, J = 9.6 Hz, 1 H), 7.40 (d, J = 12.0 Hz, 1 H), 7.22 ~ 7.32 (m, 2 H), 7.20 (d, J = 6.4 Hz, 0.09 H), 6.72 (s, 1 H), 3.74 (s, 1 H), 3.52 (s, 1 H), 2.71 ~ 2.82 (m, 4 H); 13 C NMR: 157.85, 126.97, 118.92, 93.06, 61.72.
Embodiment 3
[0024] Embodiment 3: deuterated phenylthiazine
[0025] Into a 10 mL three-necked flask, silver reagent (0.3 mmol), phosphine ligand (0.3 mmol), deuterated water (0.4 g, 20 mmol), thiothiazine (0.28 g, 1 mmol) and dimethyl sulfoxide ( 5 mL). 40 o After C reacted for 12 hours, a saturated ammonium chloride solution was added to quench the reaction, the mixed solution was extracted with dichloromethane, the organic phase was combined, the organic solvent was concentrated, and the product was further purified by column chromatography to obtain deuterated phenylthiazine with a yield of 75%. . The deuterium substitution rate is 91%. 1 H NMR: δ 7.05 ~ 7.35 (m, 4 H), 7.20 (d, J= 6.4 Hz, 0.09 H), 6.83 (s, 1 H), 3.28 ~ 3.50 (m, 2 H), 2.85 ~ 2.97 (m , 2 H),2.52 ~ 2.72 (m, 4 H), 2.25 ~ 2.44 (m, 2 H), 2.22 (s, 3 H); 13 C NMR: 157.85, 126.97, 118.92, 93.06, 61.72.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com