A kind of synthetic method of valsartan intermediate
A synthesis method and intermediate technology are applied in the field of synthesis of valsartan intermediates, can solve the problems of high consumption of n-valeryl chloride, large environmental pollution, increased consumption, etc., achieve improved purity and yield, reduced production cost, reduced The effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
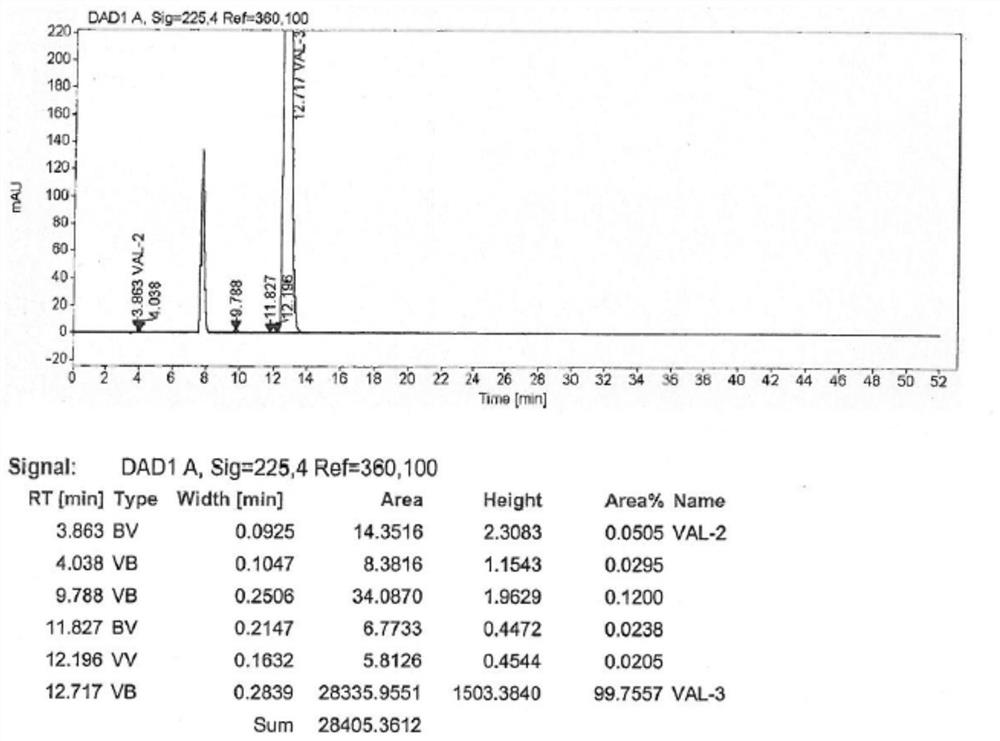
Embodiment 1
[0029] The synthetic method of the valsartan intermediate of the present embodiment comprises the following steps:
[0030] (1) add 30g formula II compound and 200mL toluene in 500mL four-necked flask, stir; 21g sodium carbonate and 18g sodium chloride are added into 120mL water, after stirring and dissolving, add dropwise to the above-mentioned four-necked flask; The temperature is 20~28℃, slowly add 12g of n-valeryl chloride and 20mL of toluene mixture dropwise, after the drop is completed, keep stirring, and detect the residue of the compound of formula II by HPLC;
[0031] (2) After HPLC detects that the residue of the compound of formula II is less than or equal to 0.10%, 120 mL of water is added, the aqueous layer is separated, the organic phase is washed with 120 mL of 2.5% ammonia water, and the aqueous layer is separated; the organic phase is washed with 50 mL of 5% aqueous hydrochloric acid, and the water is separated. The organic phase was washed with 50 mL of 2% so...
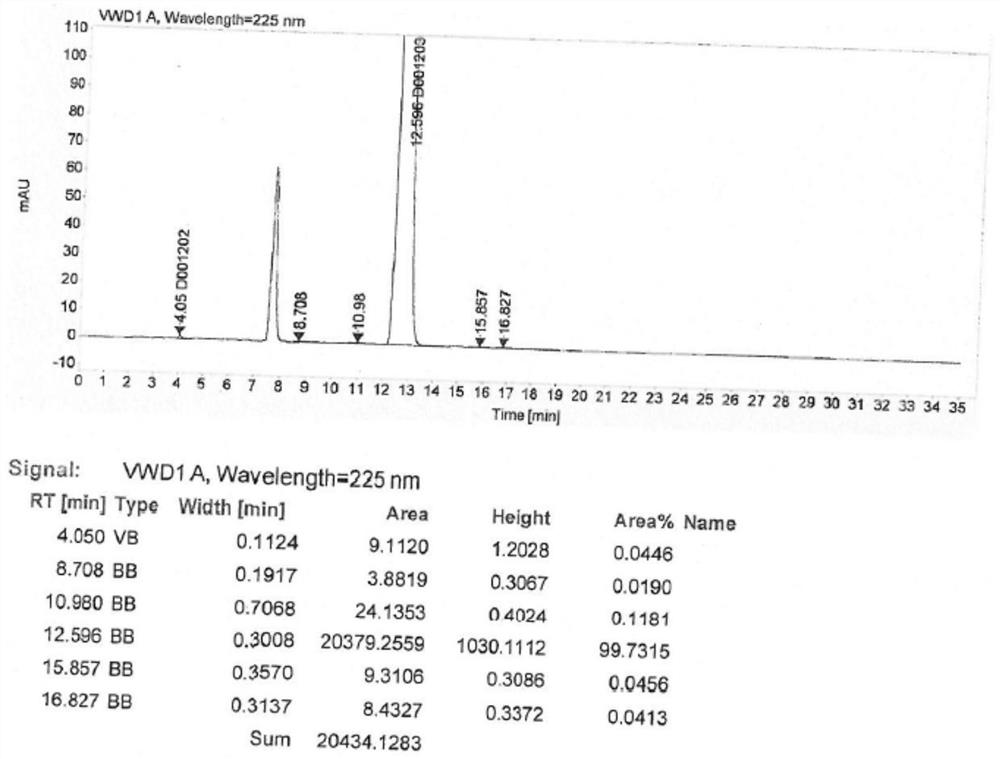
Embodiment 2
[0033] The synthetic method of the valsartan intermediate of the present embodiment comprises the following steps:
[0034] (1) in 500mL four-necked flask, add 30g formula II compound and 200mL xylene, stir; 30g potassium carbonate and 20g sodium chloride are added in 150mL water, after stirring and dissolving clear, add dropwise in above-mentioned four-necked flask; Control The inner temperature is 20~28℃, slowly add 11g n-valeryl chloride and 20mL xylene mixed solution dropwise, after dripping, keep stirring under heat, HPLC detects the residue of the compound of formula II;
[0035] (2) After HPLC detects that the residual compound of formula II is less than or equal to 0.10%, 120 mL of water is added, and the water layer is removed; the organic phase is washed with 120 mL of 2.5% ammonia water, and the water layer is removed; the organic phase is washed with 50 mL of 5% aqueous hydrochloric acid, and the water is removed. The organic phase was washed with 50 mL of 2% sodiu...
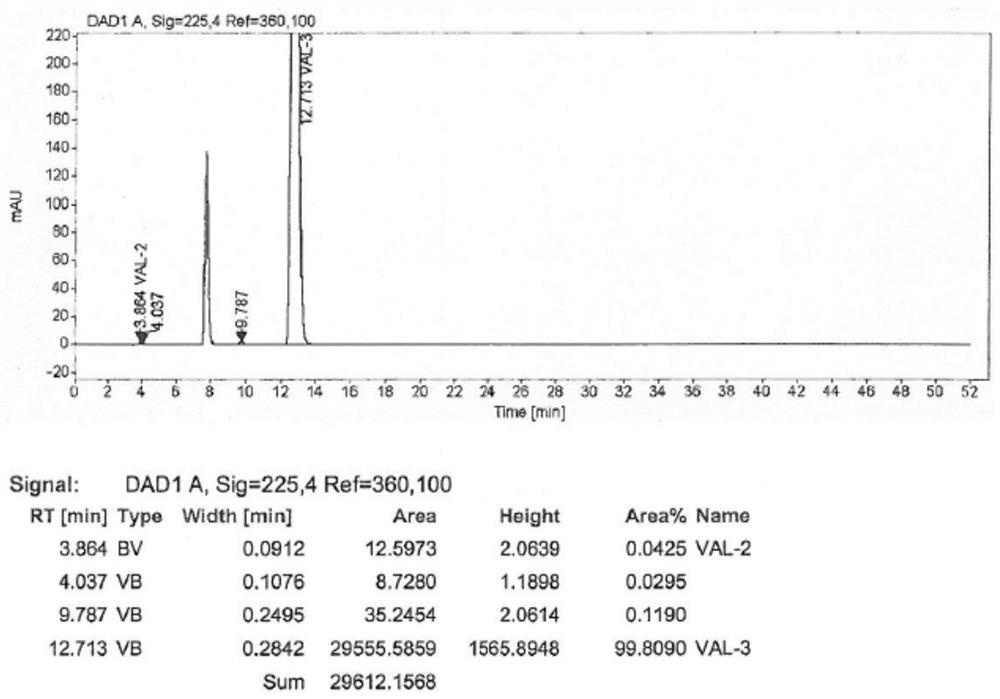
Embodiment 3
[0037] The synthetic method of the valsartan intermediate of the present embodiment comprises the following steps:
[0038] (1) in 1000mL four-necked flask, add 50g formula II compound and 335mL methylene dichloride, stir; 35g sodium carbonate and 39g potassium chloride are added in 200mL water, after stirring and dissolving clear, add dropwise in above-mentioned four-necked flask; Controlling the internal temperature at 20-28°C, slowly adding 20 g of n-valeryl chloride and 33.5 mL of dichloromethane mixture dropwise, after dripping, stirring at a temperature, HPLC detects the residue of the compound of formula II;
[0039] (2) After HPLC detects that the residue of the compound of formula II is less than or equal to 0.10%, add 200 mL of water, and remove the water layer; wash the organic phase with 200 mL of 2.5% ammonia water, and remove the water layer; wash the organic phase with 85 mL of 5% aqueous hydrochloric acid, and remove the water The organic phase was washed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com