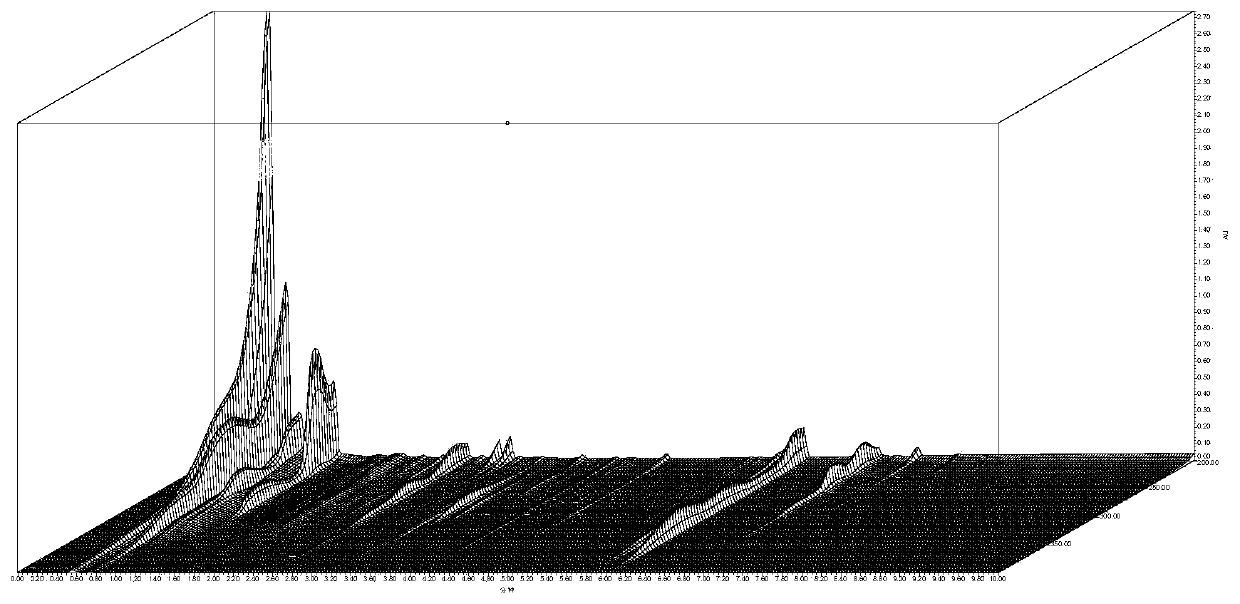
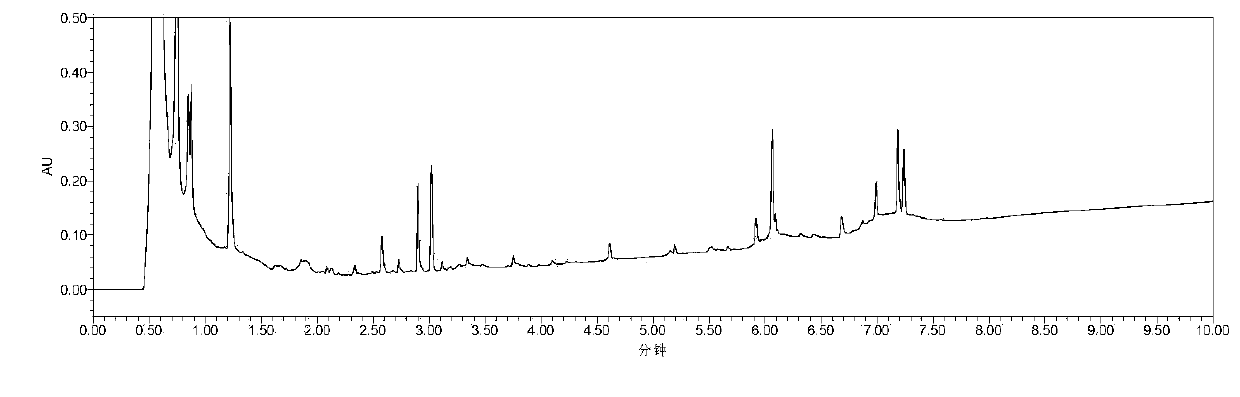
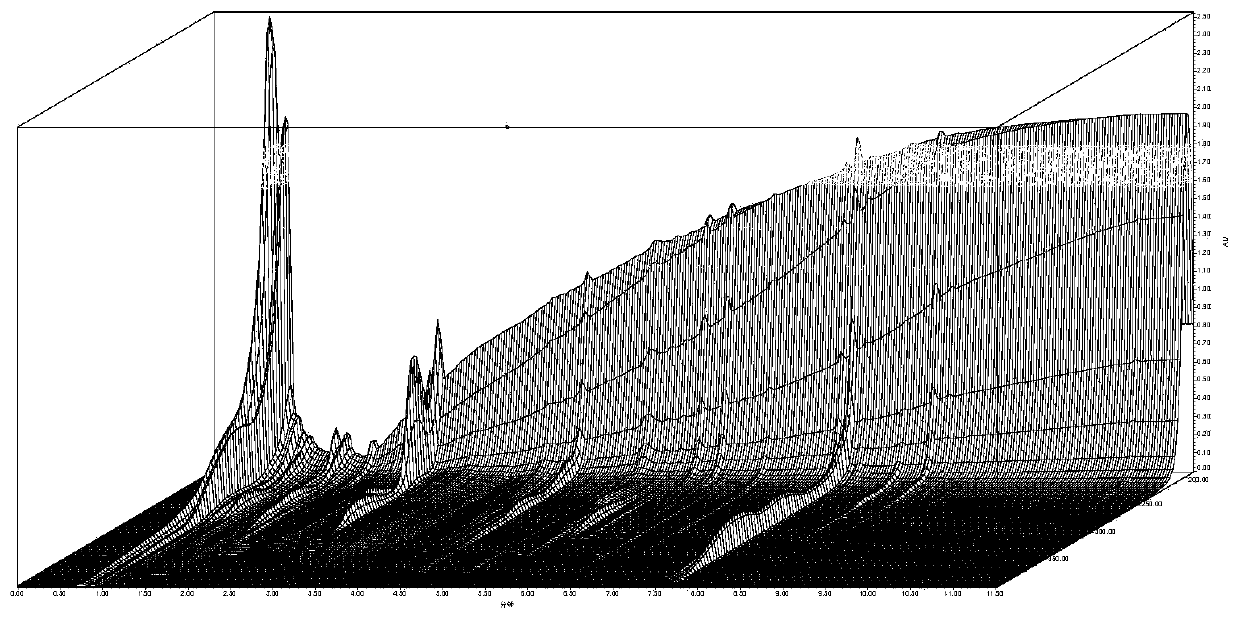
Chinese angelica standard decoction fingerprint spectrum, characteristic spectrum establishment method and content determination method
A method for establishing a fingerprint, a technique for establishing a fingerprint, applied in the establishment of a characteristic spectrum and content determination, and in the field of the fingerprint of an angelica standard decoction, achieving the effect of saving material and time costs, stable and reliable quality standards, and good separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] Preparation of Angelica Standard Decoction:
[0064] Take 150g of angelica decoction pieces, add water to decoct twice, add 9 times the amount of water to the first decoction, soak for 40 minutes, decoct for 60 minutes, filter while hot with a 400-mesh sieve, and quickly cool the filtrate with cold water. Add 7 times the amount of water for the second decoction, decoct for 40 minutes, filter while it is hot with a 400-mesh sieve, cool the filtrate quickly with cold water, and combine the two filtrates. Transfer the combined filtrate to a rotary evaporator to concentrate under reduced pressure, concentrate to an appropriate amount, and freeze-dry to obtain the angelica standard decoction.
Embodiment 1
[0065] Embodiment 1: Fingerprint is established
[0066] 1. Investigation on Chromatographic Conditions of Angelica Standard Decoction
[0067] 1. Chromatographic conditions and system suitability test
[0068] Accurately draw 5 μl of the test solution respectively, inject it into a liquid chromatograph, measure, record the chromatogram, and obtain it. Table 1 shows the initial conditions of the fingerprint of Angelica Standard Decoction, and Table 2 shows the mobile phase gradient table. Determine according to high performance liquid chromatography ("Chinese Pharmacopoeia" 2015 edition four general rules 0512).
[0069] Table 1 The initial conditions of the fingerprints of Angelica standard decoction
[0070]
[0071]
[0072] Table 2 Initial mobile phase gradient table
[0073] time (minutes) Mobile phase A(%) Mobile phase B(%) 0 10 90 7 90 10 10 100 0
[0074] (1) Selection of detection wavelength
[0075] On the basis of the ...
Embodiment 2
[0142] The establishment of embodiment 2 feature map
[0143] Chromatographic conditions: according to the final experimental conditions proposed in Example 1.
[0144] Preparation of the reference substance solution: take an appropriate amount of the reference substance of ferulic acid, weigh it accurately, put it in a brown measuring bottle, add methanol to make a solution containing 12 μg per 1 ml, and obtain it.
[0145] Determination method: Precisely draw 1 μl of the reference substance solution and 5 μl of the test product solution respectively, inject it into the liquid chromatograph, measure, record the chromatogram, and obtain it.
[0146] One, the establishment of the method for preparing the test solution
[0147] 1. Selection of extraction solvent
[0148] Take about 0.15g of this product (batch number B01), weigh it precisely, put it into a stoppered Erlenmeyer flask, add methanol, 50% methanol, and 25ml of water precisely, shake well, weigh, ultrasonicate for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com