Biomimetic polymer containing dopamine or dopamine-like functional group and preparation method thereof
A dopamine and polymer technology, applied in the field of biomimetic polymers and their preparation, can solve the problems of waste of raw materials, unsuitable for industrial production, and long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
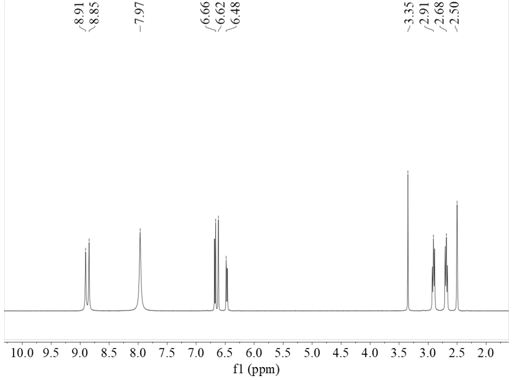
[0043] (A) Synthesis of Modified Methacrylamide Monomer: Take 10gNa 2 B 4 O 7 , 4gNaHCO 3 Add it to the reaction eggplant bottle, then add 100ml ultrapure water, then add 5g II-19; then dissolve 4.7ml methacrylic anhydride in 25ml THF, add dropwise to the above solution; then configure 1M NaOH, add dropwise In the above-mentioned solution, adjust pH 8~9, carry out nitrogen magnetic stirring reaction at room temperature for 17 hours; add 50 ml of ethyl acetate to the reaction solution after the reaction is completed, let stand for stratification, take the lower water phase, repeat 2 times; 6M HCl was added dropwise to the filtrate to adjust pH=2; then the filtrate was transferred to a round-bottomed flask, 50 ml of ethyl acetate was added, and extracted 3 times; finally, it was precipitated into cold 500 ml of n-hexane, filtered with suction, and the filter residue was taken and dried 5.6 g of the modified methacrylamide monomer (III-1) was obtained with a yield of 82%.
[...
Embodiment 2
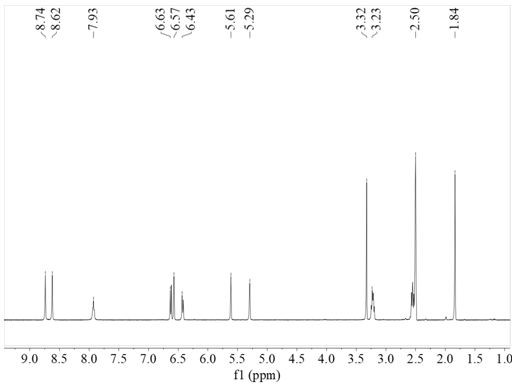
[0046] (A) Synthesis of Modified Methacrylamide Monomer: Take 10gNa 2 B 4 O 7 , 4gNaHCO 3 Add it to the reaction eggplant bottle, then add 100ml ultrapure water, then add 5g II-2; then dissolve 4.7ml methacrylic anhydride in 25ml THF, add dropwise to the above solution; then configure 1M NaOH, add dropwise In the above-mentioned solution, adjust pH 8~9, carry out nitrogen magnetic stirring reaction at room temperature for 17 hours; add 50 ml of ethyl acetate to the reaction solution after the reaction is completed, let stand for stratification, take the lower water phase, repeat 2 times; 6M HCl was added dropwise to the filtrate to adjust pH=2; then the filtrate was transferred to a round-bottomed flask, 50 ml of ethyl acetate was added, and extracted 3 times; finally, it was precipitated into cold 500 ml of n-hexane, filtered with suction, and the filter residue was taken and dried 5.6 g of the modified methacrylamide monomer (III-2) was obtained with a yield of 80%.
[0...
Embodiment 3
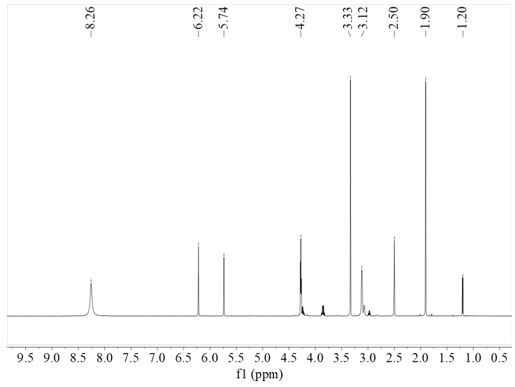
[0049] (A) Synthesis of Modified Methacrylamide Monomer: Take 10gNa 2 B 4 O 7 , 4gNaHCO 3 Add it to the reaction eggplant bottle, then add 100ml ultrapure water, then add 5.5g II-3; then dissolve 4.7ml methacrylic anhydride in 25ml THF, add it dropwise to the above solution; then configure 1M NaOH, dropwise was added to the above solution, adjusted to pH 8-9, and reacted with nitrogen magnetic stirring at room temperature for 17 hours; after the reaction was completed, 50 ml of ethyl acetate was added to the reaction solution, left to stand for stratification, and the lower water phase was taken out and repeated twice; Then add 6M HCl dropwise to the filtrate to adjust pH=2; then transfer the filtrate to a round-bottomed flask, add 50 ml of ethyl acetate, and extract 3 times; After drying, 6.1 g of the modified methacrylamide monomer (III-3) was obtained in a yield of 81%.
[0050] (B) Polymerization: photo-initiated polymerization, 0.0125 g (0.05 mmol) of thiopropoxythiox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com