Viable cell mitochondria G-quadruplex targeted fluorescent probe and application thereof
A fluorescent probe and cell technology, applied in the field of analytical chemistry, can solve problems such as influence, and achieve the effects of small damage, low cytotoxicity, and easy detection and operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 1. The synthesis methods of compounds (1), (2) and (6) are as follows, wherein, the reaction ratio and purification method adopt conventional ratios or conventional purification methods in the art. In addition, the inventors have confirmed that the structures of the above-mentioned compounds are correct by analyzing the hydrogen spectrum, carbon spectrum and / or mass spectrum data of each compound, wherein the compound (1) 1 H-NMR spectra and mass spectra were referred to Figure 9 and Figure 10 .
[0074]
[0075] 2. The synthesis methods of compounds (3) and (4) are as follows, wherein the reaction ratios and purification methods adopt conventional ratios or conventional purification methods in the art. In addition, the inventors have confirmed that the structures of the above compounds are correct by analyzing the hydrogen spectrum, carbon spectrum and / or mass spectrum data of each compound.
[0076]
[0077] 3. The synthesis method of compound (5) is as fol...
Embodiment 2
[0080] Utilize fluorescent probe 1 of the embodiment of the present invention to carry out cytotoxicity experiment, specifically as follows:
[0081]
[0082] fluorescent probe 1
[0083] (1) Dissolve fluorescent probe 1 with a small amount of methanol;
[0084] (2) Add different concentrations of probe 1 solutions to the cultured HeLa and MCF-7 cells, and continue to culture for 24 hours;
[0085] (3) Add 10% MTT solution after sucking up the culture medium, and continue culturing for 4 hours;
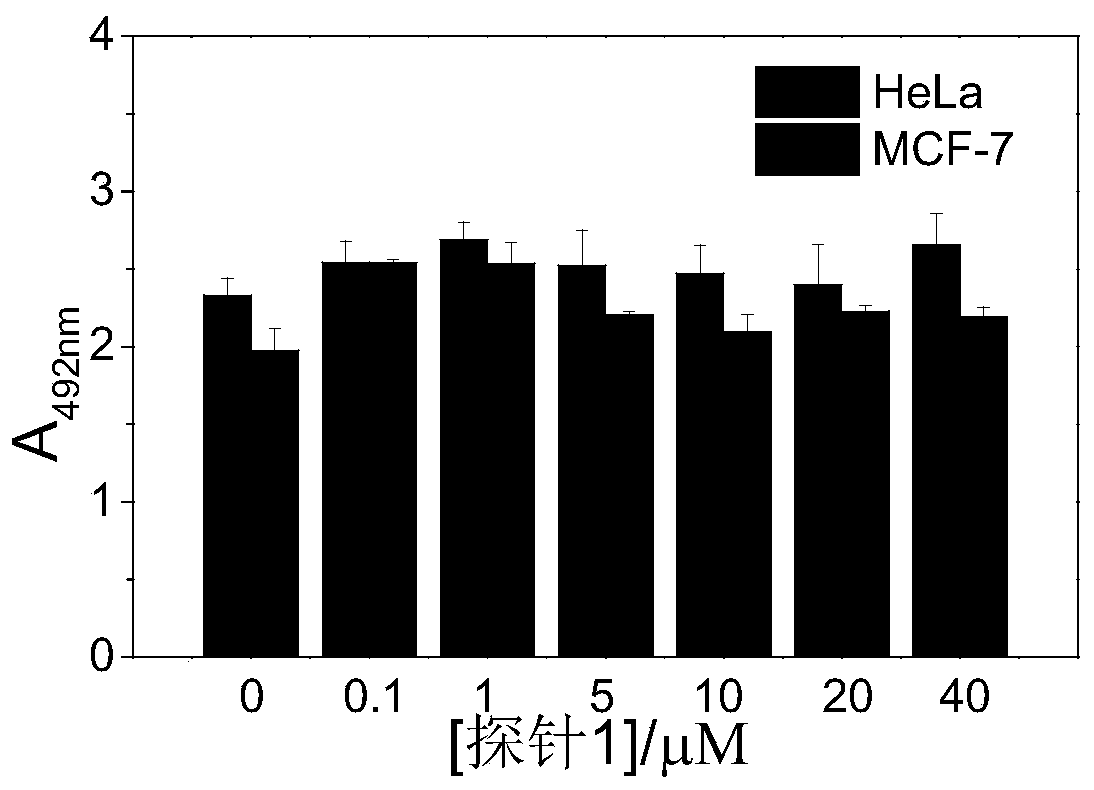
[0086] (4) After the culture medium was blotted dry, DMSO was added to dissolve it, and the absorbance at 492 nm was measured with a microplate reader. The absorbance value at 492nm is plotted on the ordinate, and the concentration value of probe 1 is on the abscissa. The result is as figure 1 As shown, the absorbance value at 492nm has no significant difference at different probe 1 concentrations, indicating that probe 1 has no inhibitory effect on cell growth.
Embodiment 3
[0088] Utilize the fluorescent probe 1 of the embodiment of the present invention to carry out the pH interference experiment, specifically as follows:
[0089]
[0090] fluorescent probe 1
[0091] (1) Dissolve fluorescent probe 1 with a small amount of ethanol;
[0092] (2) Add the fluorescent probe solution in step (1) to phosphate buffer solutions with different pH values, and the concentration of the probe in each sample solution is 20 μM;
[0093] (3) Place each sample solution in a HITACHI F-4600 fluorescence spectrometer (Hitachi Limited, Japan) for detection, the excitation wavelength is 550nm, and the collection wavelength is 560-700nm;
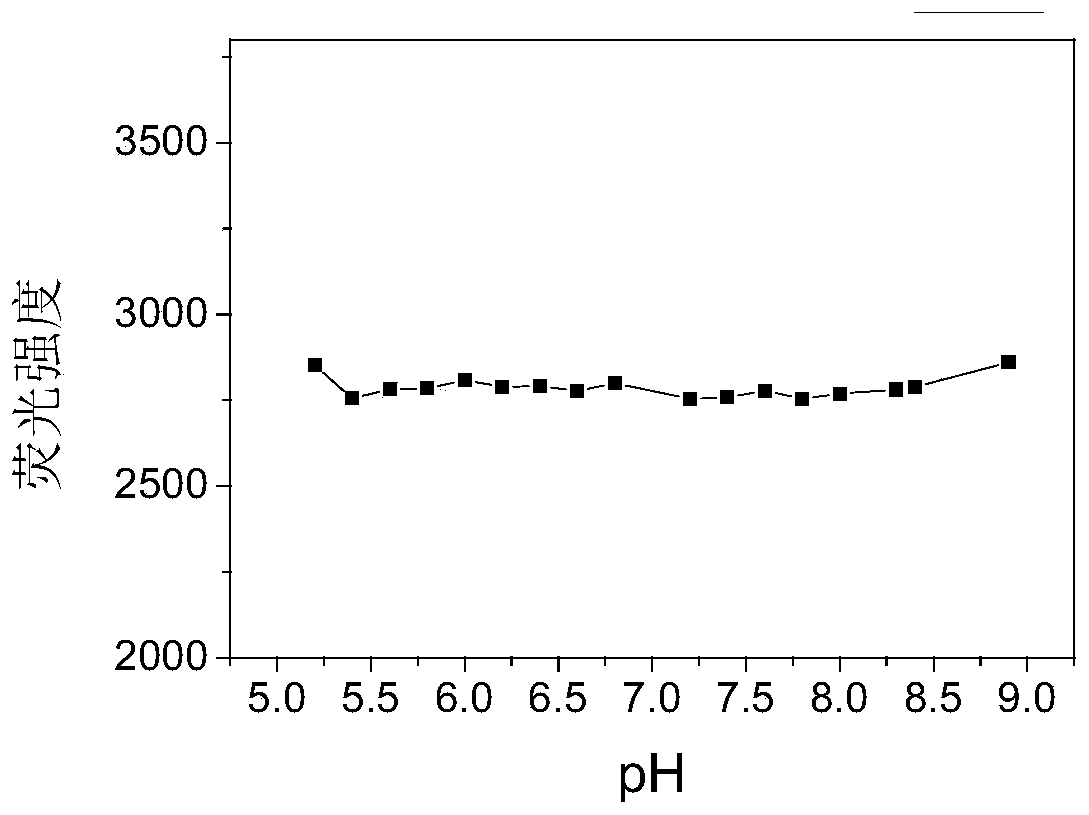
[0094] (4) Get the maximum fluorescence intensity as the ordinate, and pH as the abscissa for analysis, such as figure 2 shown. At each pH value, the fluorescence intensity values are similar, indicating that the fluorescence of probe 1 is not easily affected by pH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com