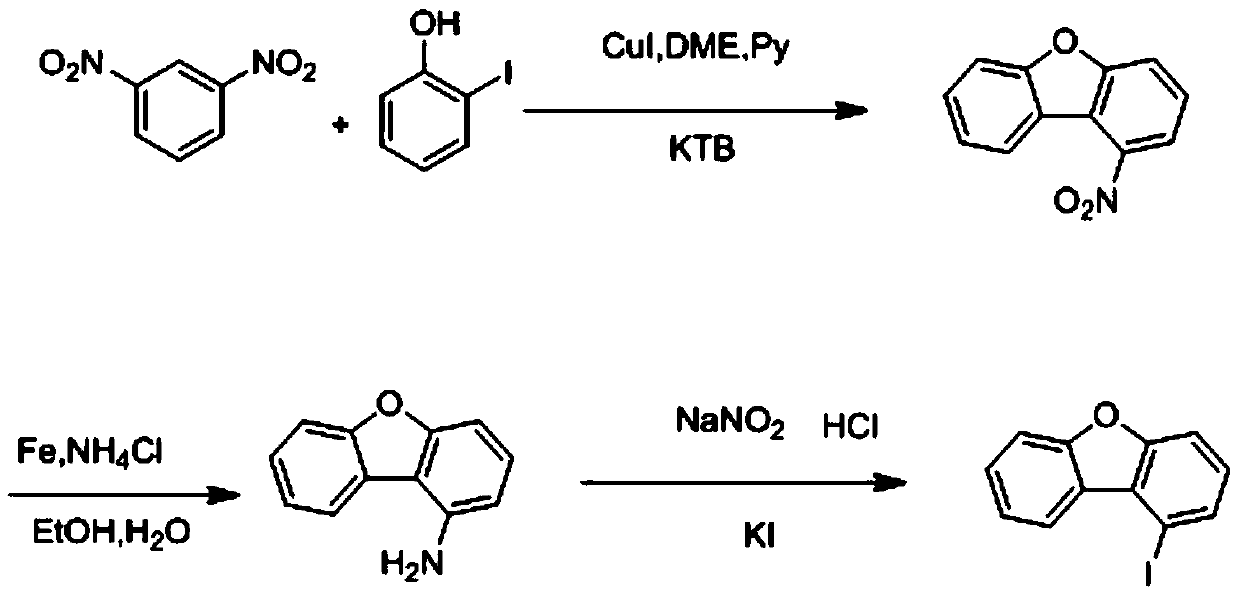
Preparation method of 1-iododibenzofuran
A technology of nitrodibenzofuran and aminodibenzofuran, which is applied in the field of preparation of 1-iododibenzofuran, can solve the problems of high synthesis cost, many reaction raw materials, long reaction time, etc., and achieve stable yield , The synthesis method is simple and easy, and the effect of little pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
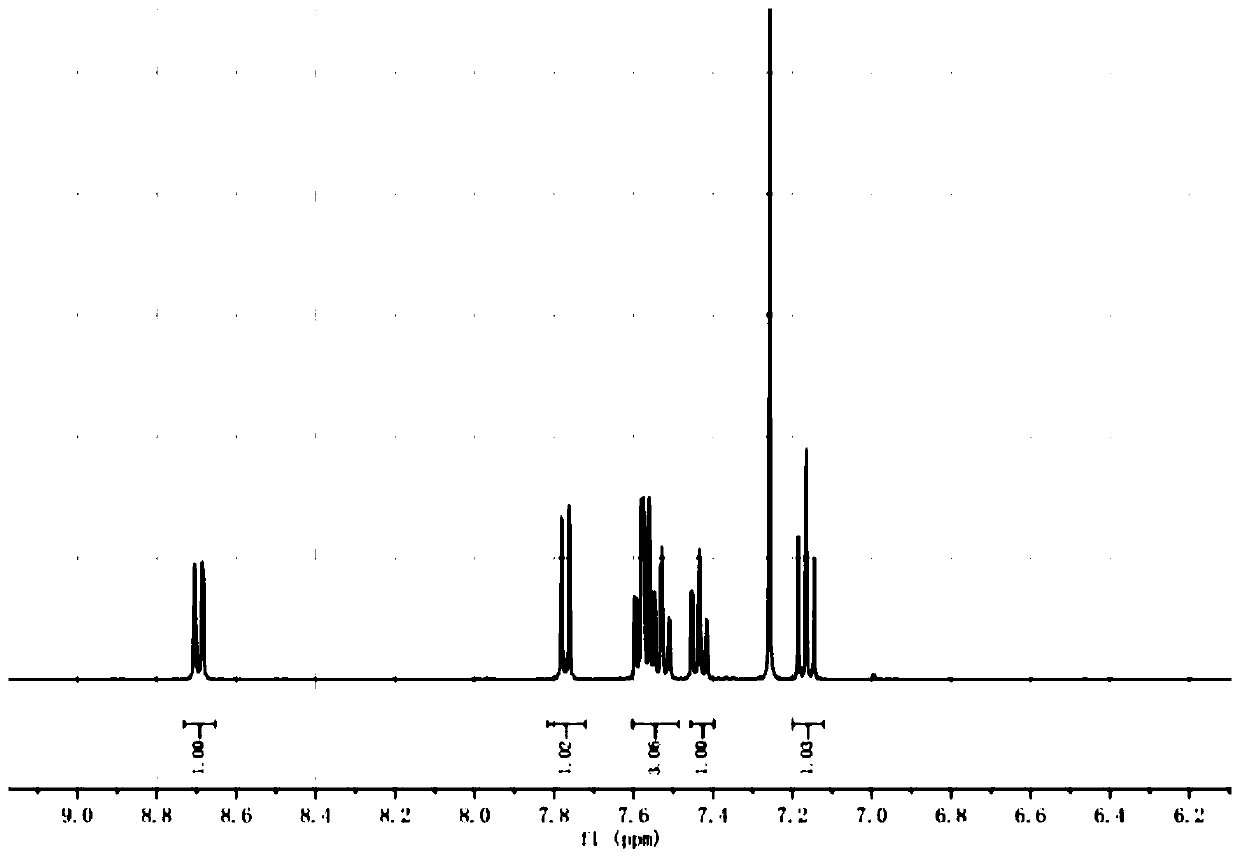
Image
Examples
Embodiment 1
[0023] A preparation method of 1-iododibenzofuran, comprising the following steps:
[0024] S1. In a 500mL round bottom flask, add dimethyl ether (66.4mL), potassium tert-butoxide (13.3g, 0.119mol), cuprous iodide (11.32g, 0.058mol), replace with nitrogen three times, and stir for 1h , Dissolve m-dinitrobenzene (6.64g, 0.039mol) with pyridine (66.4mL), add it into a round bottom flask, and stir for 1h, potassium tert-butoxide (5.3g, 0.047mol) and o-iodophenol (10g, 0.045mol) was dissolved with dimethyl ether (20mL), the temperature needs to be controlled below 10°C, and then added to the system, after nitrogen replacement, the external temperature was set to 100°C, and the reaction was carried out for 7 hours. Use a spot plate to observe the completion of the reaction, and then add 1mol / L hydrochloric acid After washing with water and suction filtration, the filtrate was extracted with dimethyl ether (65mL), concentrated to 10mL, petroleum ether (10mL) was added, silica gel (1...
Embodiment 2
[0028] A preparation method of 1-iododibenzofuran, comprising the following steps:
[0029] S1. In a 100L reactor, add dimethyl ether (13.28L), potassium tert-butoxide (2.66Kg, 23.72mol), cuprous iodide (2.25Kg, 11.86mol), nitrogen replacement three times, and stir for 1h. Dissolve m-dinitrobenzene (1.33Kg, 7.91mol) with pyridine (13.3L), add to the reaction kettle, stir for 1h, potassium tert-butoxide (1.064Kg, 9.49mol) and o-iodophenol (2Kg, 9.09mol ) is dissolved in dimethyl ether (6.63L), and the temperature needs to be controlled below 10°C, and then added to the system. After nitrogen replacement, the external temperature is set to 100°C, and the reaction is carried out for 7 hours. Use a spot plate to observe the completion of the reaction, and then add 1mol / L hydrochloric acid to wash. , after suction filtration, the filtrate was extracted with dimethyl ether (13L), concentrated to 2L, and petroleum ether (2L) was added, and then silica gel (1Kg) was added for adsorpti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com