Novel c-Met/HDAC double-target inhibitor, and synthetic method and application thereof
A synthesis method and technology of c-met are applied in the field of medicine to achieve the effects of improving utilization efficiency and therapeutic effect, simple preparation method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
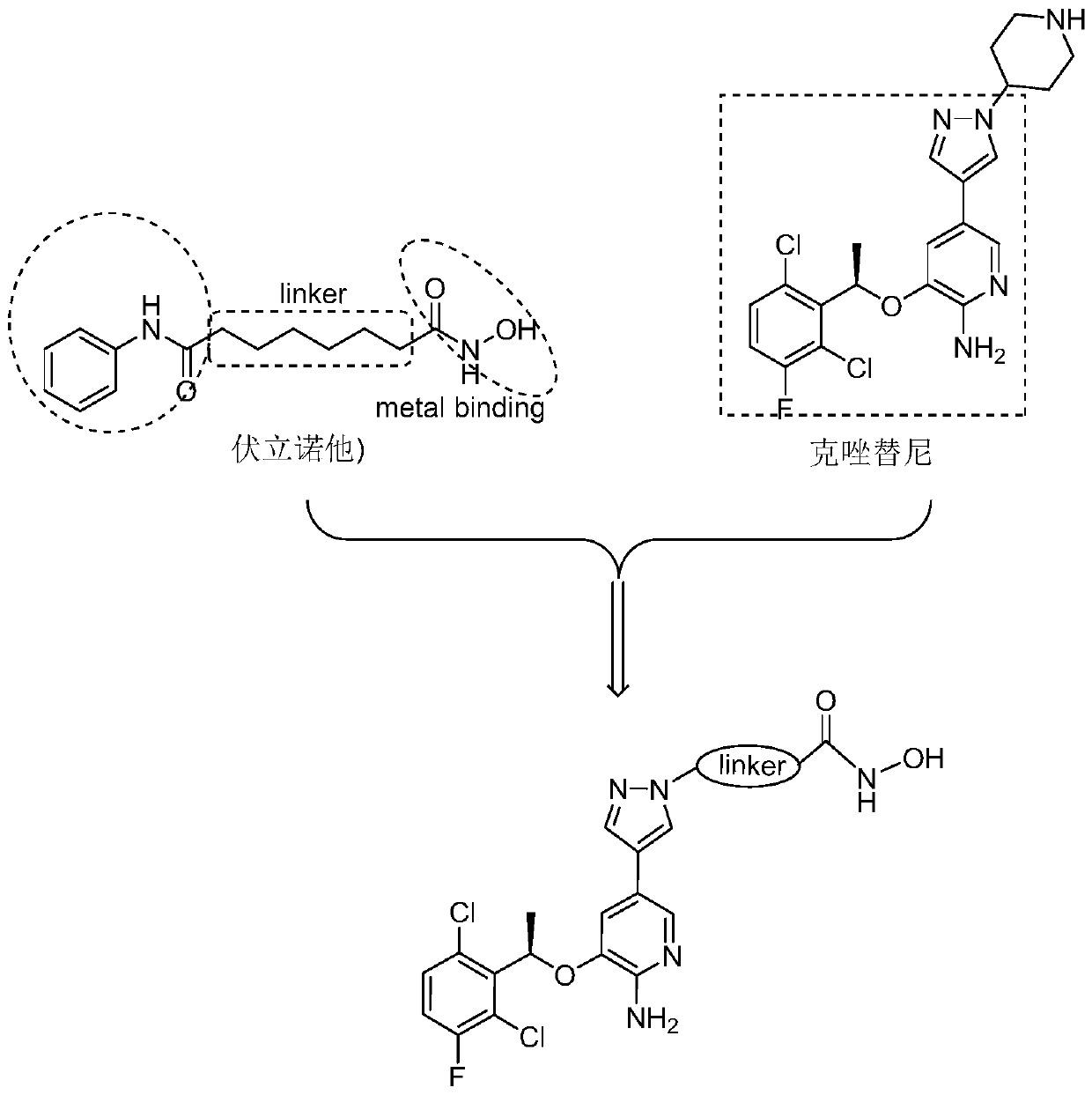
[0042] The structural formula of the novel c-Met / HDAC dual-target inhibitor in this example is as follows:
[0043]
[0044] Among them, the linker is n=2.
[0045] The synthesis method of the novel c-Met / HDAC dual-target inhibitor in this example is as follows:
[0046] (1) at first synthetic intermediate (a) reaction equation is as follows:
[0047]
[0048] At room temperature, 4-pyrazoleboronic acid pinacol ester (3.9g, 20.0mmol) was dissolved in 30.0mlDMF, potassium carbonate (5.5g, 40.0mmol) and 3-bromopropionate methyl ester (4.0g, 24.0mmol), after stirring at room temperature for 24 hours, TLC monitoring (the volume ratio of petroleum ether to ethyl acetate is 2:1) the reaction raw material still remains. Add 30ml of water to the reaction mixture, stir until clear liquid, then extract with ethyl acetate (50ml×2), wash the extract with saturated brine (50ml×2), dry over anhydrous sodium sulfate, filter, and depressurize the filtrate After concentration, the r...
Embodiment 2
[0063] In the structural formula of the c-Met / HDAC dual-target inhibitor in this example, the linker is n=3, synthesized according to the method of Example 1, replacing methyl 3-bromopropionate with methyl 4-bromobutyrate, and adjusting other test parameters routinely to obtain the target product (I-2), the 1HNMR data is as follows :
[0064] 1HNMR (400MHz, DMSO-d6):ppm: 10.43(s,1H),7.88(s,1H),7.74(d,J=1.68Hz,1H),7.57(dd,J=4.76Hz,4.76Hz,1H ),7.53(s,1H),7.45(t,J=8.68Hz,8.68Hz 1H),6.89(s,1H),6.06-6.11(q,J=6.72Hz,1H),5.68(s,2H) , 4.06 (t, J = 6.72Hz, 5.92Hz, 2H), 1.92-1.99 (m, 4H), 1.79 (d, J = 6.44Hz, 3H).
Embodiment 3
[0066] In the structural formula of the c-Met / HDAC dual-target inhibitor in this example, the linker is n=4, synthesized according to the method of Example 1, replacing methyl 3-bromopropionate with methyl 5-bromovalerate, and routinely adjusting other test parameters to obtain the target product (I-3), the 1HNMR data is as follows :
[0067] 1 HNMR (400MHz, DMSO-d 6 ):ppm:10.38(s,1H),8.78(s,1H),7.87(s,1H),7.73(d,J=1.68Hz,1H),7.57(dd,J=4.76Hz,5.04Hz,1H ),7.52(s,1H),7.44(t,J=8.96Hz,8.86Hz,1H),6.88(d,J=1.44Hz,1H),6.06-6.11(q,J=6.88Hz,6.72Hz, 6.72Hz, 1H), 5.68(s, 2H), 4.06(t, J=6.72Hz, 7Hz, 2H), 1.97(t, J=7.32Hz, 7.28Hz, 2H), 1.80(d, J=6.72Hz ,3H), 1.69-1.76(m,2H), 1.40-1.47(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com