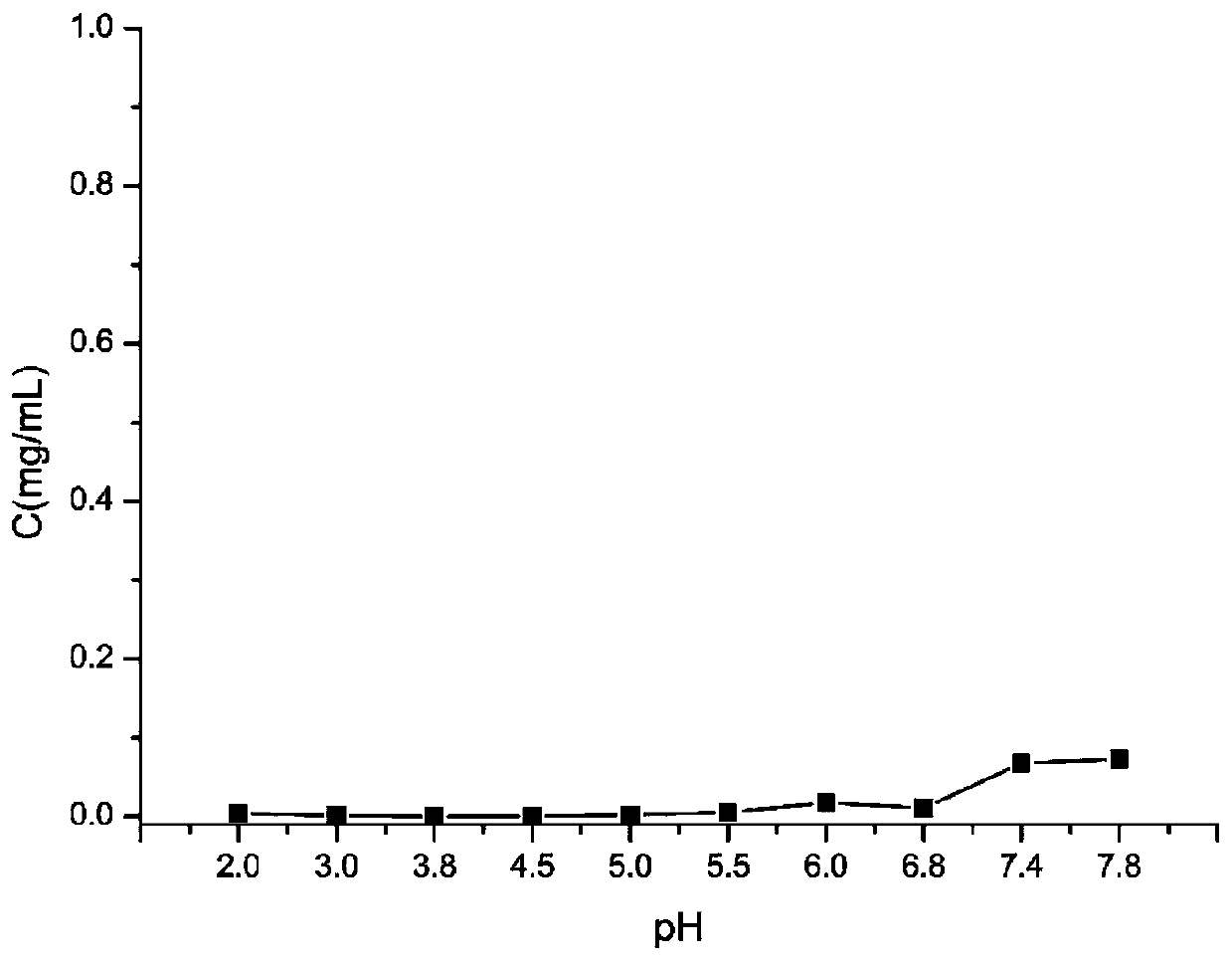
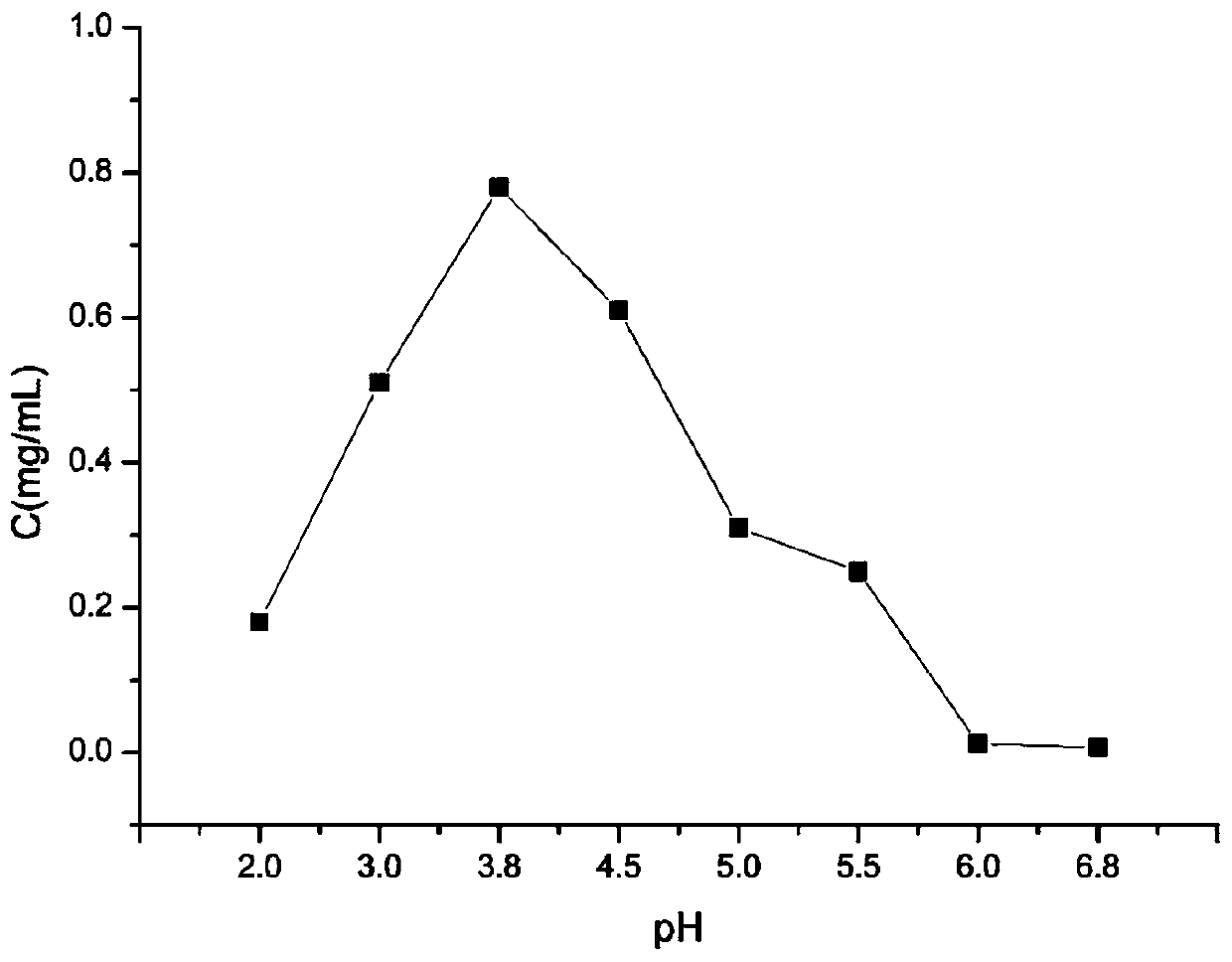
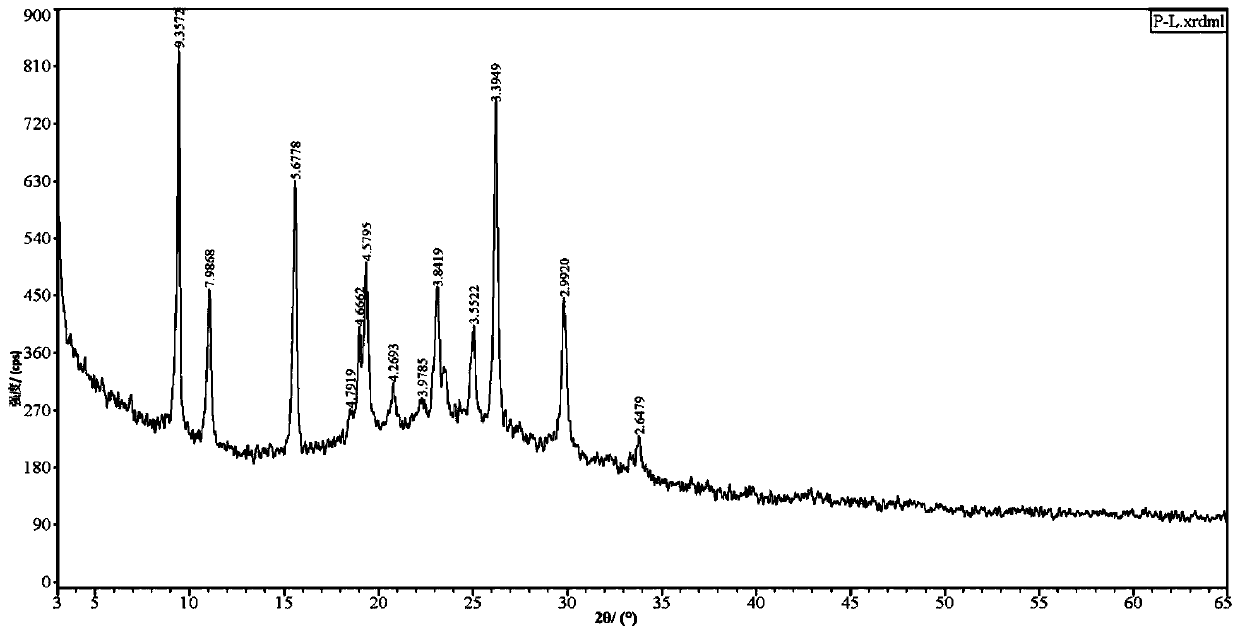
Lurasidone crystal, preparation method thereof and application of lurasidone crystal in injection drug delivery system
A technology for lurasidone pamoate and crystals, applied in the field of medicine, can solve the problems of large differences, non-compliance with pH and no dependence, undisclosed solubility of lurasidone hydrochloride crystals, and the like, so as to improve solubility and solubility. The absolute change value is small and the effect of increasing the drug load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of pamoic acid lurasidone crystal of the present invention comprises the following steps:
[0053] (1) dissolving lurasidone and pamoic acid in tetrahydrofuran to obtain the first mixed solution;
[0054] (2) adding ethyl acetate to the first mixed solution, stirring, heating and reacting and then cooling down to room temperature to obtain a second mixed solution;
[0055] (3) After spinning the second mixed solution to dryness, ethyl acetate was added, dichloromethane crystallized, and the crystals were precipitated and filtered to obtain lurasidone pamoic acid crystals.
[0056] In step (1), the molar ratio of lurasidone and pamoic acid can be 1:1-1.2, most preferably 1:1.
[0057] In step (2), preferably, heating to 55-60° C. for 2-3 hours.
[0058] The preparation method of pamoic acid lurasidone nanocrystal of the present invention comprises the following steps:
[0059] (a) preparing pamoic acid lurasidone crystals according to the above ...
Embodiment 1
[0075] This example is used to illustrate the preparation method of pamoic acid lurasidone crystals.
[0076] Dissolve 1g of lurasidone and 788mg of pamoic acid in 20ml of tetrahydrofuran, then add 20ml of ethyl acetate, react at 60°C for 2h and then spin dry. Add 10ml of ethyl acetate and 5ml of dichloromethane, and filter to obtain the product lurasidone pamoate A1 after crystallization.
Embodiment 2
[0078] This example is used to illustrate the preparation method of pamoic acid lurasidone crystals.
[0079] Dissolve 1g of lurasidone and 788mg of pamoic acid in 30ml of tetrahydrofuran, then add 10ml of ethyl acetate, react at 55°C for 3h and spin dry. Add 15ml of ethyl acetate and 8ml of dichloromethane, and filter to obtain the product lurasidone pamoate A2 after crystallization. The product was vacuum-dried at 50°C, and stored in a glass bottle at room temperature, dry, and protected from light.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com