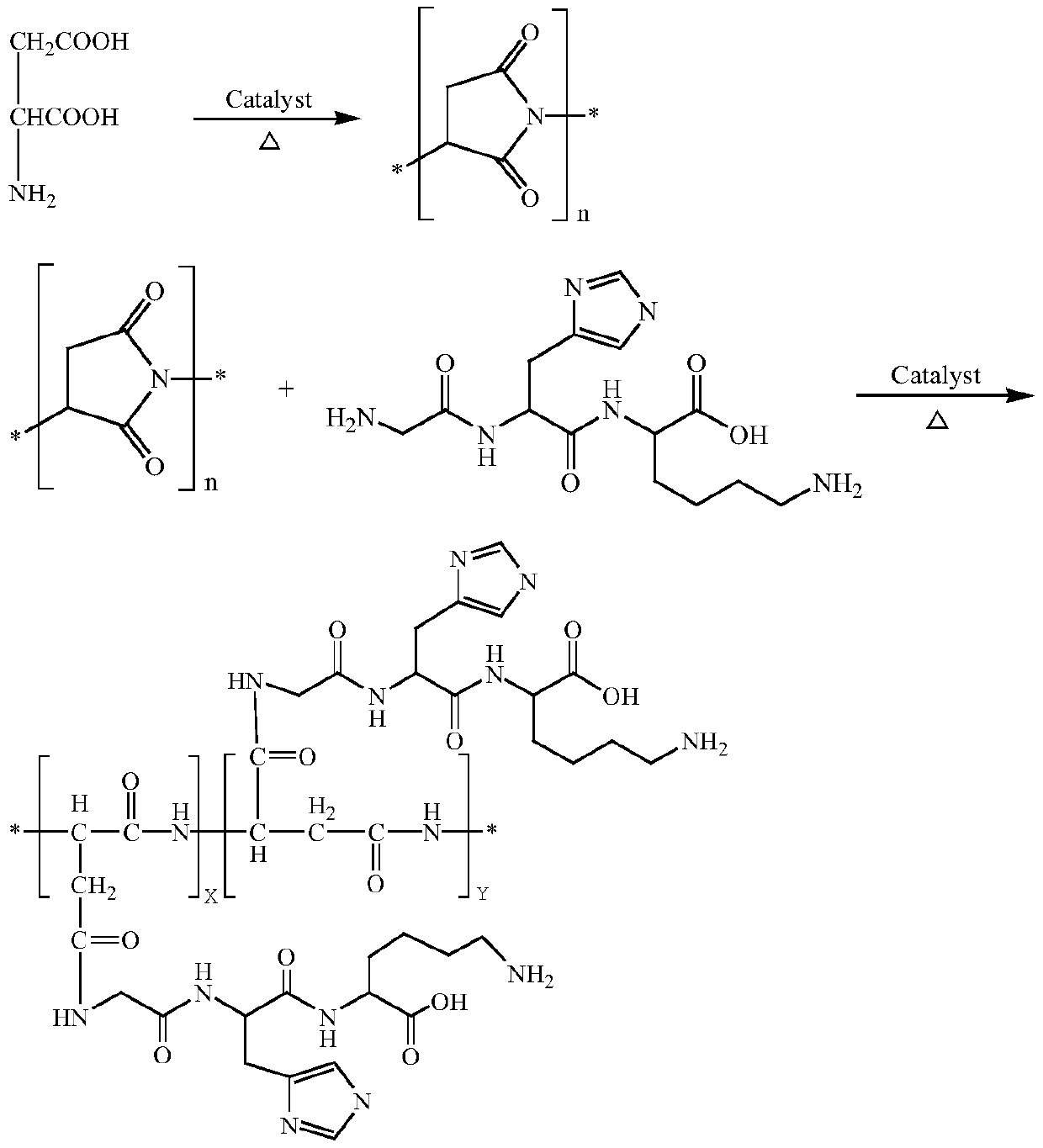
Synthetic method for glycyl-histidyl-lysine grafted polyaspartic acid derivative
A technology of glycyl histidyl lysine acetate and glycyl histidyl lysine is applied in the field of preparation of polyaspartic acid derivatives, and can solve the problem of limited functionality and application limitations of polyamino acids and other problems, to achieve the effect of simple and green synthesis steps, stimulating skin tissue repair, and simplifying process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Purification of glycyl histidyl lysine: choose 50 parts of crude glycyl histidyl lysine synthesized by liquid phase method and dissolve it in 500 parts of ultrapure water, and dissolve it with ultrasonic wave. After the solution is completely clarified, use Filter through a 0.35 μm microporous membrane and collect the filtrate. The obtained filtrate uses a strong cation exchange column to remove trifluoroacetic acid and some impurities, and converts it into an acetate solution. The obtained acetate solution was desalted and purified using a reverse-phase polymer column, and finally the target fraction was collected, concentrated by distillation under reduced pressure, and freeze-dried to obtain pure glycyl-histidyl-lysine.
[0028] Synthesis of polyaspartic acid salt aqueous solution grafted with glycyl histidyl lysine: add 100 parts of L-aspartic acid into the kneading reactor, raise the temperature to 175°C, and control it under the condition of vacuum degree -20kPa ...
Embodiment 2
[0030] Purification of glycyl histidyl lysine: choose 40 parts of crude glycyl histidyl lysine synthesized by liquid phase method and dissolve it in 400 parts of ultrapure water, and ultrasonically assist the dissolution. After the solution is completely clarified, use Filter through a 0.45 μm microporous membrane and collect the filtrate. The obtained filtrate uses a strong cation exchange column to remove trifluoroacetic acid and some impurities, and converts it into an acetate solution. The obtained acetate solution was desalted and purified using a reverse-phase polymer column, and finally the target fraction was collected, concentrated by distillation under reduced pressure, and freeze-dried to obtain pure glycyl-histidyl-lysine.
[0031] Synthesis of polyaspartic acid salt aqueous solution grafted with glycyl histidyl lysine: add 200 parts of L-aspartic acid into the kneading reactor, raise the temperature to 185°C, and control it under the condition of vacuum degree -35...
Embodiment 3
[0033] Purification of glycyl histidyl lysine: select 30 parts of crude glycyl histidyl lysine synthesized by liquid phase method and dissolve it in 300 parts of ultrapure water, and dissolve it with ultrasonic wave. After the solution is completely clarified, use Filter through a 0.45 μm microporous membrane and collect the filtrate. The obtained filtrate uses a strong cation exchange column to remove trifluoroacetic acid and some impurities, and converts it into an acetate solution. The obtained acetate solution was desalted and purified using a reverse-phase polymer column, and finally the target fraction was collected, concentrated by distillation under reduced pressure, and freeze-dried to obtain pure glycyl-histidyl-lysine.
[0034] Synthesis of polyaspartic acid salt aqueous solution grafted with glycyl histidyl lysine: add 120 parts of L-aspartic acid into the kneading reactor, raise the temperature to 195°C, and control it under the condition of vacuum degree -50kPa ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com