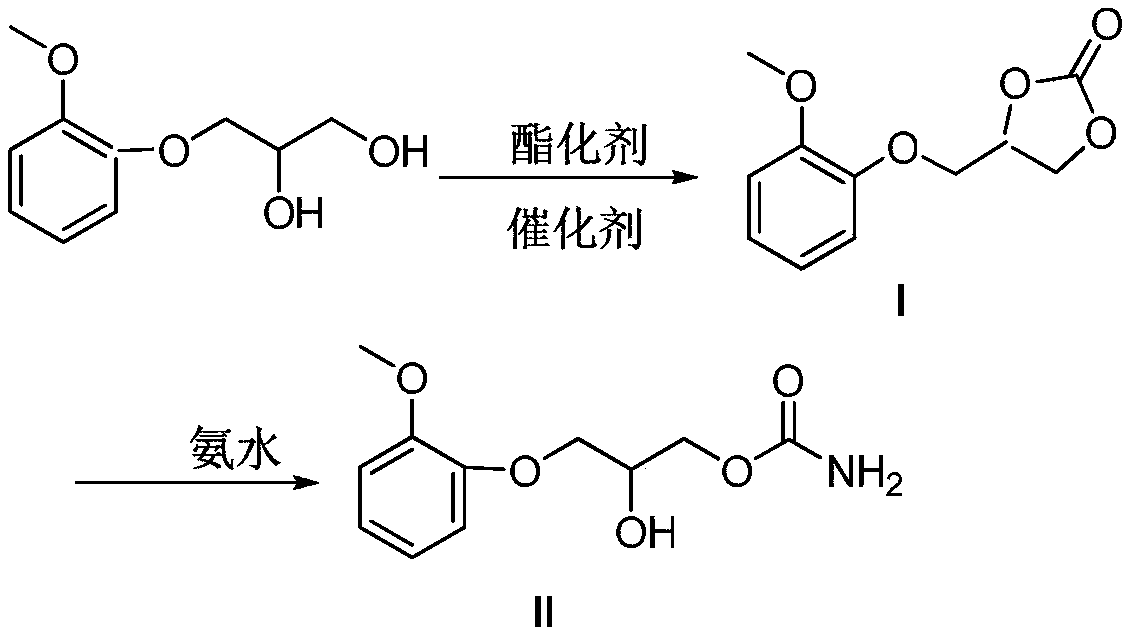
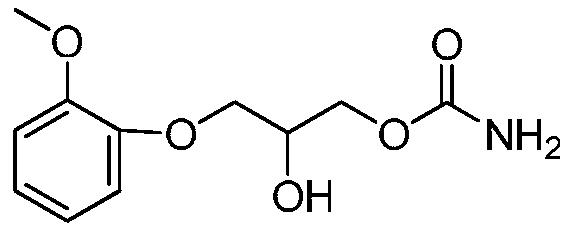
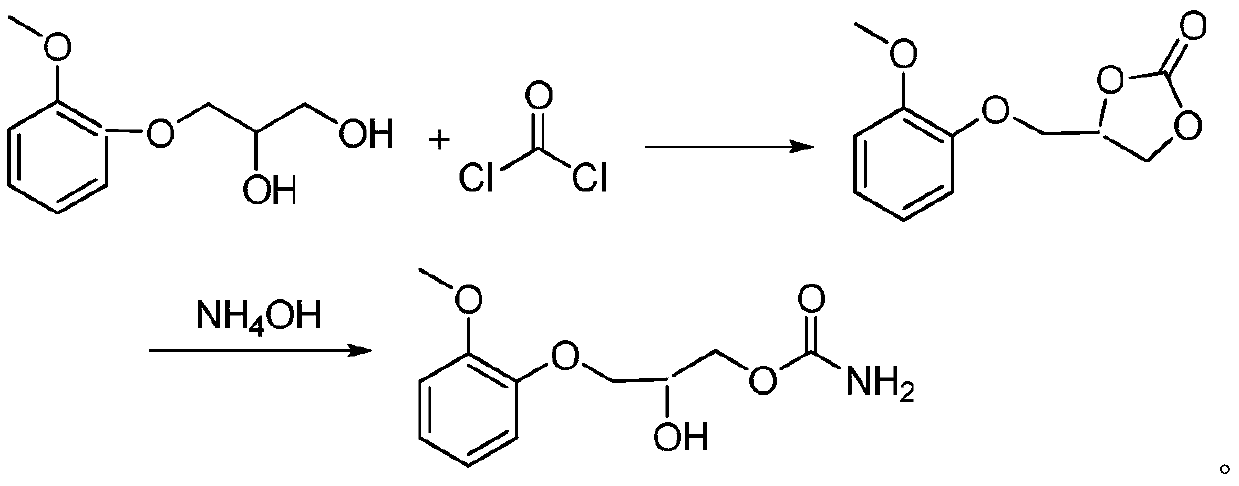
Method for preparing methocarbamol
A technology of methocarbamol and carbonate, which is applied in the field of chemical synthesis, can solve the problems of low economic efficiency and large amount of dimethyl carbonate, and achieve the effects of good economic benefits, less pollution, and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of methocarbamol
[0037] Add guaiacol glycerin ether 40g (0.2mol), lithium hydroxide 0.2g (0.0083mol), DMAP 0.1g (0.00082mol) in 500ml reaction bottle, warm up to 80 ℃ and add dropwise dimethyl carbonate 22g (0.24mol ), the dropwise addition was completed and refluxed for 4 hours, and TLC followed the reaction process. After the reaction, cool to 40°C, add 34g (0.4mol) of 20% ammonia solution, and react at this temperature for 6 hours to obtain crude methocarbamol, which is recrystallized with 100ml of pure water to obtain 45.6g, yield 95% .
Embodiment 2
[0038] Embodiment 2: the preparation of methocarbamol
[0039] Add guaiacol glycerin ether 40g (0.2mol), sodium hydroxide 0.32g (0.008mol), DMAP 0.1g (0.00082mol) in 500ml reaction bottle, warm up to 80 ℃ and add dropwise dimethyl carbonate 22g (0.24mol ), the dropwise addition was completed and refluxed for 4 hours, and TLC followed the reaction process. After the reaction, cool to 40°C, add 34g (0.4mol) of 20% ammonia solution, and react at this temperature for 6 hours to obtain crude methocarbamol, which is recrystallized with 100ml of pure water to obtain 44.6g, with a yield of 93%. .
Embodiment 3
[0040] Embodiment 3: the preparation of methocarbamol
[0041] Add guaiacol glycerin ether 40g (0.2mol), potassium hydroxide 0.45g (0.008mol), DMAP 0.1g (0.00082mol) in 500ml reaction bottle, warm up to 80 ℃ and add dropwise dimethyl carbonate 22g (0.24mol ), the dropwise addition was completed and refluxed for 4 hours, and TLC followed the reaction process. After the reaction, cool to 40°C, add 34g (0.4mol) of 20% ammonia solution, and react at this temperature for 6 hours to obtain crude methocarbamol, which is recrystallized with 100ml of pure water to obtain 43.2g, yield 90% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com