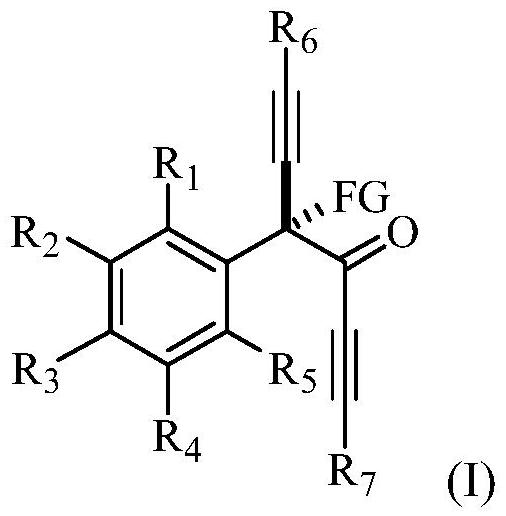
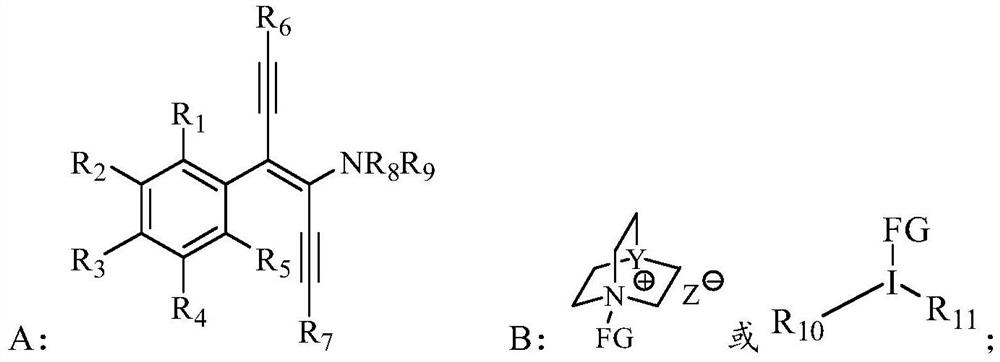
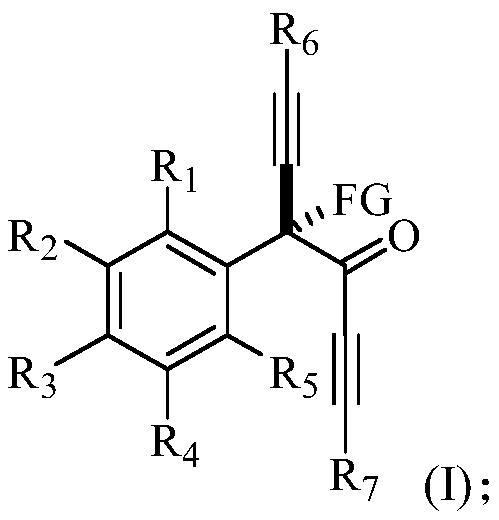
α-tetrasubstituted chiral acetylenone compound and its preparation method and application
A compound and tetra-substitution technology, applied in the field of chiral compound synthesis, can solve the problems of harsh preparation conditions, unfavorable subsequent transformation of products, and complicated processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] This example provides a chiral R-4-fluoro-4-phenyl-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyn-3-one and its preparation method . The structural formula of the R-4-fluoro-4-phenyl-1,6-bis(triisopropylsilyl) n-hexane-1,5-diyn-3-one is shown in the following molecular structural formula I1:
[0117]
[0118] Its preparation steps are as follows:
[0119] Add homo-S-3,3'-bis(2,4,6-triisopropylphenyl)-6,6'-dinitro-binaphthyl phosphate (0.005mmol, 0.1 eq), sodium carbonate (0.10mmol, 2.0eq), 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt (0.06mmol, 1.2 eq) and 0.5 mL of anhydrous m-xylene, replaced with argon three times, sealed the reaction test tube and stirred at room temperature for 10 min, then placed it in a -30°C cold trap. (R,trans)-3-tert-butyldiphenylsilyloxy-1-(4-phenyl-1,6-bis(triisopropylsilyl)n-hexyl-3-ene-1, A solution of 5-diyne-3-)pyrrolidine (0.05mmol, 1.00eq) in m-xylene was slowly added to the reaction system, an...
Embodiment 2
[0121] This example provides a chiral R-4-fluoro-4-(m-methylphenyl)-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyne-3- Ketones and methods for their preparation. The structural formula of the R-4-fluoro-4-(m-methylphenyl)-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyn-3-one is as follows molecular structure formula I2 Show:
[0122]
[0123] Its preparation steps are as follows:
[0124] Add homo-S-3,3'-bis(2,4,6-triisopropylphenyl)-6,6'-dinitro-binaphthyl phosphate (0.005mmol, 0.1 eq), sodium carbonate (0.10mmol, 2.0eq), 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt (0.06mmol, 1.2 eq) and 0.5 mL of anhydrous m-xylene, replaced with argon three times, sealed the reaction test tube and stirred at room temperature for 10 min, then placed it in a -30°C cold trap. (R,trans)-3-tert-butyldiphenylsilyloxy-1-(4-m-methylphenyl-1,6-bis(triisopropylsilyl)n-hexyl-3-ene The m-xylene solution of -1,5-diyne-3-)pyrrolidine (0.05mmol, 1.00eq) was slowly ...
Embodiment 3
[0126] This example provides a chiral R-4-fluoro-4-(p-methylphenyl)-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyne-3- Ketones and methods for their preparation. The structural formula of the R-4-fluoro-4-(p-methylphenyl)-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyn-3-one is shown in molecular structural formula I3 Show:
[0127]
[0128] Its preparation steps are as follows:
[0129] Add homo-S-3,3'-bis(2,4,6-triisopropylphenyl)-6,6'-dinitro-binaphthyl phosphate (0.005mmol, 0.1 eq), sodium carbonate (0.10mmol, 2.0eq), 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt (0.06mmol, 1.2 eq) and 0.5 mL of anhydrous m-xylene, replaced with argon three times, sealed the reaction test tube and stirred at room temperature for 10 min, then placed it in a -30°C cold trap. (R,trans)-3-tert-butyldiphenylsilyloxy-1-(4-p-methylphenyl-1,6-bis(triisopropylsilyl)n-hexyl-3-ene The m-xylene solution of -1,5-diyne-3-)pyrrolidine (0.05mmol, 1.00eq) was slowly a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com