Fluorescent material, preparation method and application
A technology of fluorescent materials and electron-donating groups, which is applied in the field of electroluminescent materials, can solve the problems of low luminous efficiency of luminescent materials, and achieve the effects of reducing luminescence quenching process, increasing yield, and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
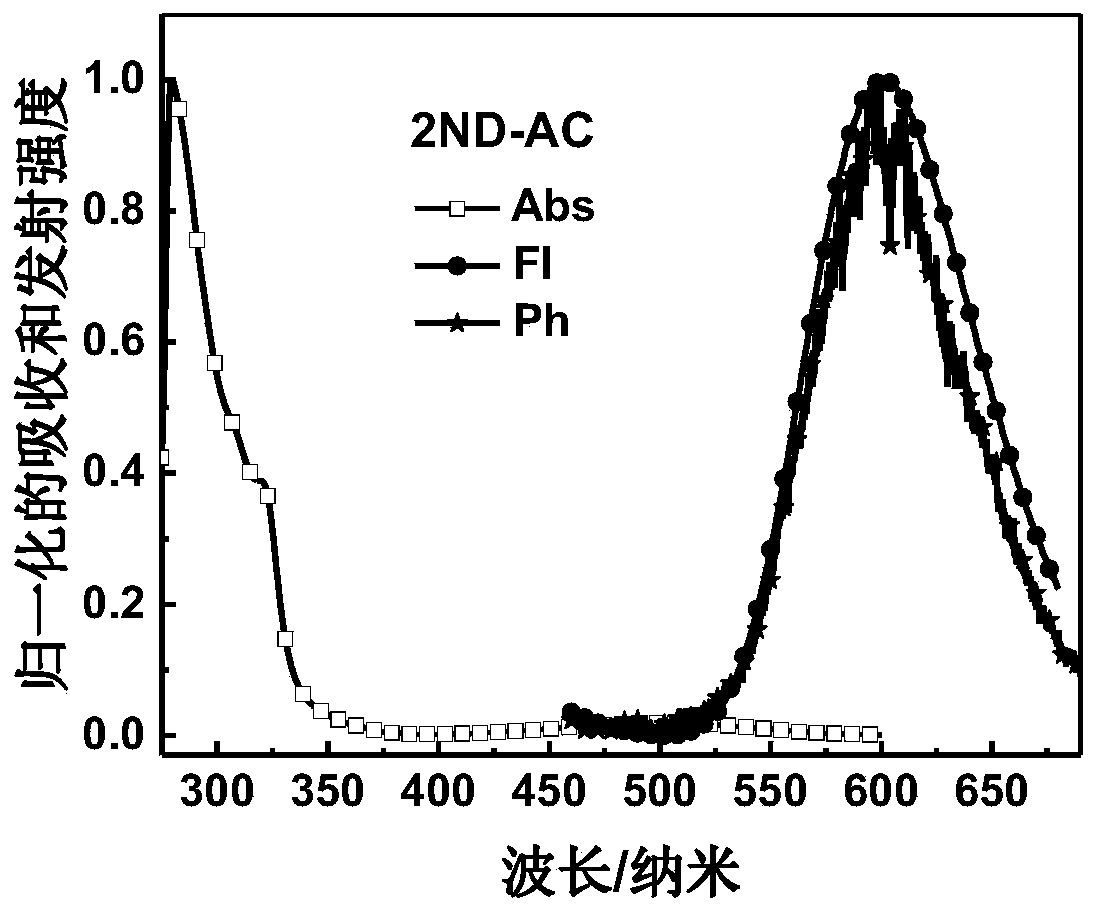
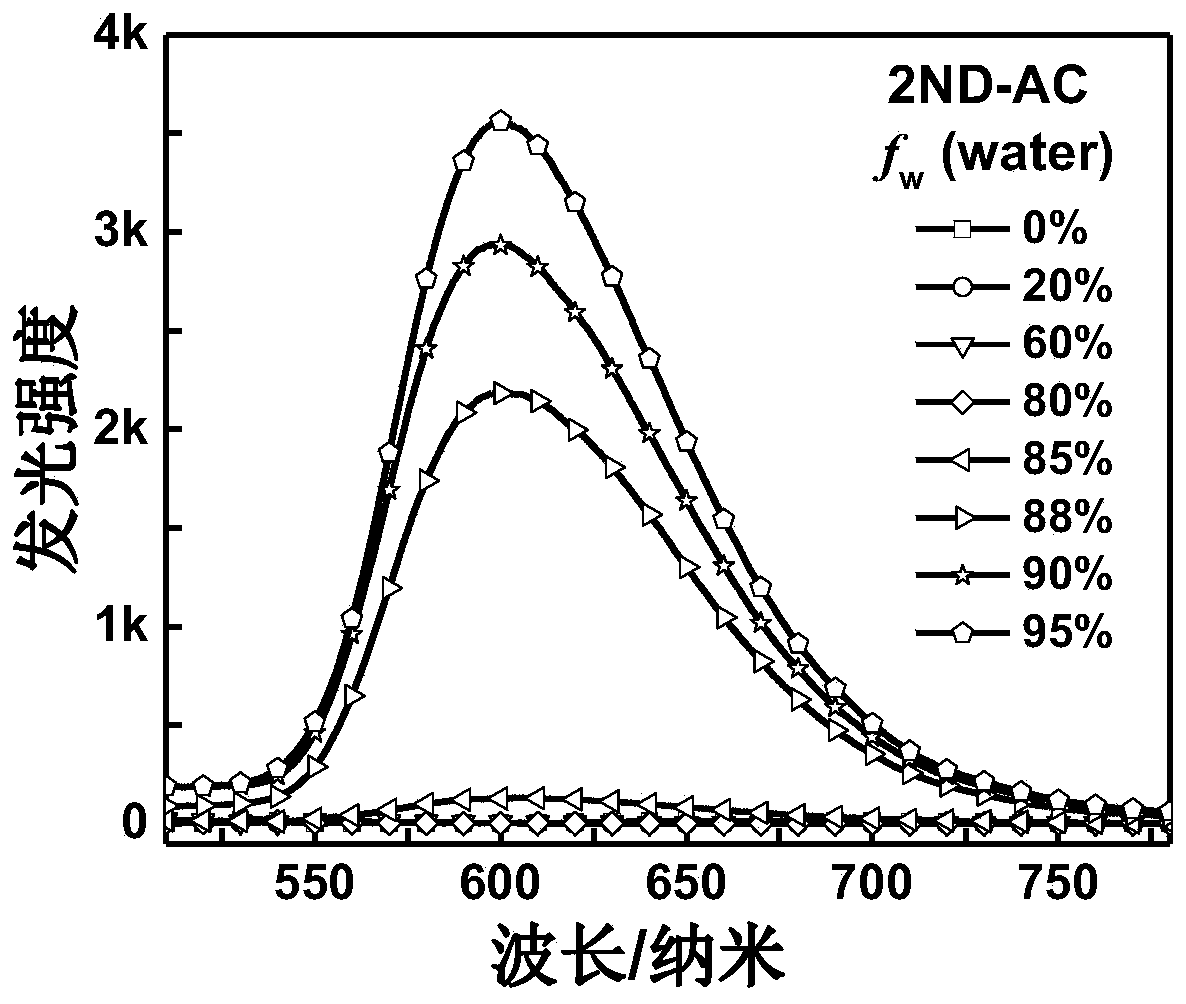
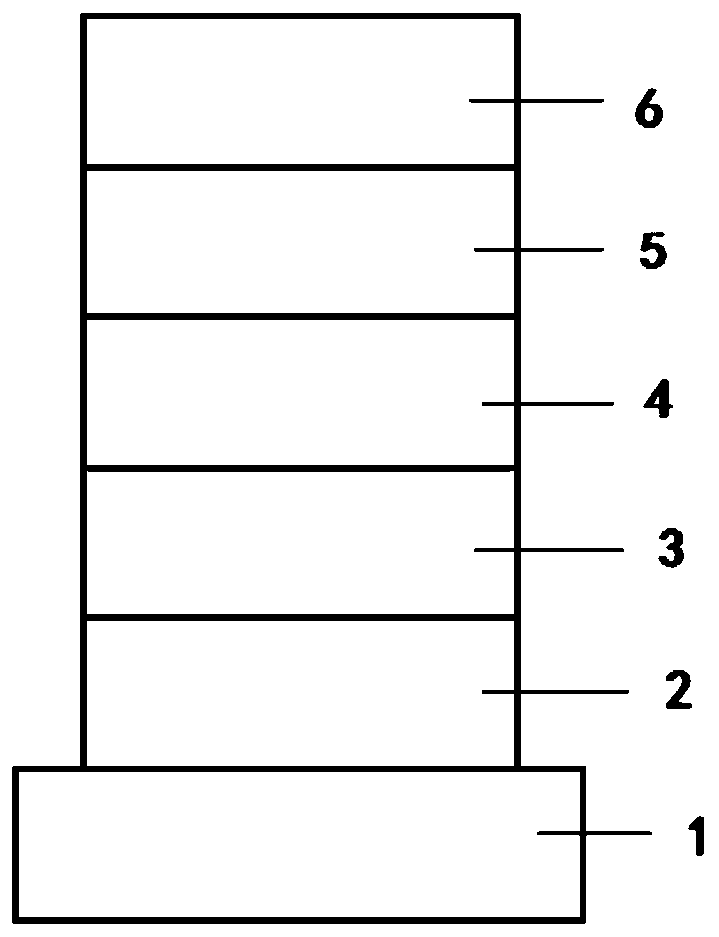
Image
Examples
preparation example Construction
[0044] The present invention also provides a preparation method for the above-mentioned fluorescent material, including:
[0045] Step A, preparation of intermediate E containing naphthyridine unit:
[0046] Specifically, intermediate E can adopt the following synthetic route.
[0047]
[0048] In the formula, X and Y are not H at the same time, and the synthesis steps are: disperse compound A and compound B in an organic solvent, for example, using toluene as a solvent, reflux for more than 10 minutes under a protective atmosphere (argon can be used), and separate the compound C; then compound C is placed in diphenyl ether, refluxed for 4-8 hours, separated and purified to obtain compound D; then compound D is placed in phosphorus oxychloride, and the catalyst N,N-dimethylaniline is added to protect Reflux under atmosphere for 1-4 hours, separate and purify to obtain the intermediate E.
[0049]Step B, step B, under anhydrous and oxygen-free conditions, disperse the in...
Embodiment 1
[0061] Compound of Example 1 preparation.
[0062] (1) Synthesis of intermediate 8-A
[0063]
[0064] In a 250 mL two necked round bottom flask, 3-amino-4 chloropyridine (2.56 g, 20 mmol), dibutyl maleate (2.88 g, 20 mmol) and 100 mL of toluene were added. The mixture was refluxed and stirred for 15 minutes under an argon atmosphere, cooled to room temperature, and 250 mL of petroleum ether was added, resulting in a large amount of precipitation, which was filtered by suction and dried to obtain 4.16 g of a light yellow solid with a yield of 92%.
[0065] (2) Synthesis of Intermediate 8-B
[0066] In a 250mL round bottom flask, add Intermediate 8-A (4.07g, 18mmol) and 130mL of diphenyl ether, the mixture is refluxed at 255°C for 5 hours under argon atmosphere, filtered with suction, and the solid is washed with dichloromethane to obtain a brown Crude product 1.62g, yield 50%.
[0067] (3) Synthesis of intermediate ND-2Cl
[0068] In a 50mL two-necked round-bottomed f...
Embodiment 2
[0081] Example 2: Compounds preparation.
[0082] (1) Synthesis of Intermediate 9-A
[0083]
[0084] In a 250 mL two necked round bottom flask, 3-fluoro-5-amino-4 chloropyridine (2.92 g, 20 mmol), ethyl ethoxymethylene fluoroacetate (3.24 g, 20 mmol) and 100 mL of toluene were added. The mixture was refluxed and stirred for 15 minutes under an argon atmosphere, cooled to room temperature, and 250 mL of petroleum ether was added, resulting in a large amount of precipitation, which was filtered by suction and dried to obtain 4.82 g of a light yellow solid with a yield of 92%.
[0085] (2) Synthesis of Intermediate 9-B
[0086] In a 250mL round bottom flask, add Intermediate 9-A (4.71g, 18mmol) and 130mL of diphenyl ether, the mixture is refluxed at 260°C for 5 hours under argon atmosphere, filtered with suction, and the solid is washed with dichloromethane to obtain brown Crude product 1.94g, yield 50%.
[0087] (3) Synthesis of intermediate FND-2Cl
[0088] In a 50mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com