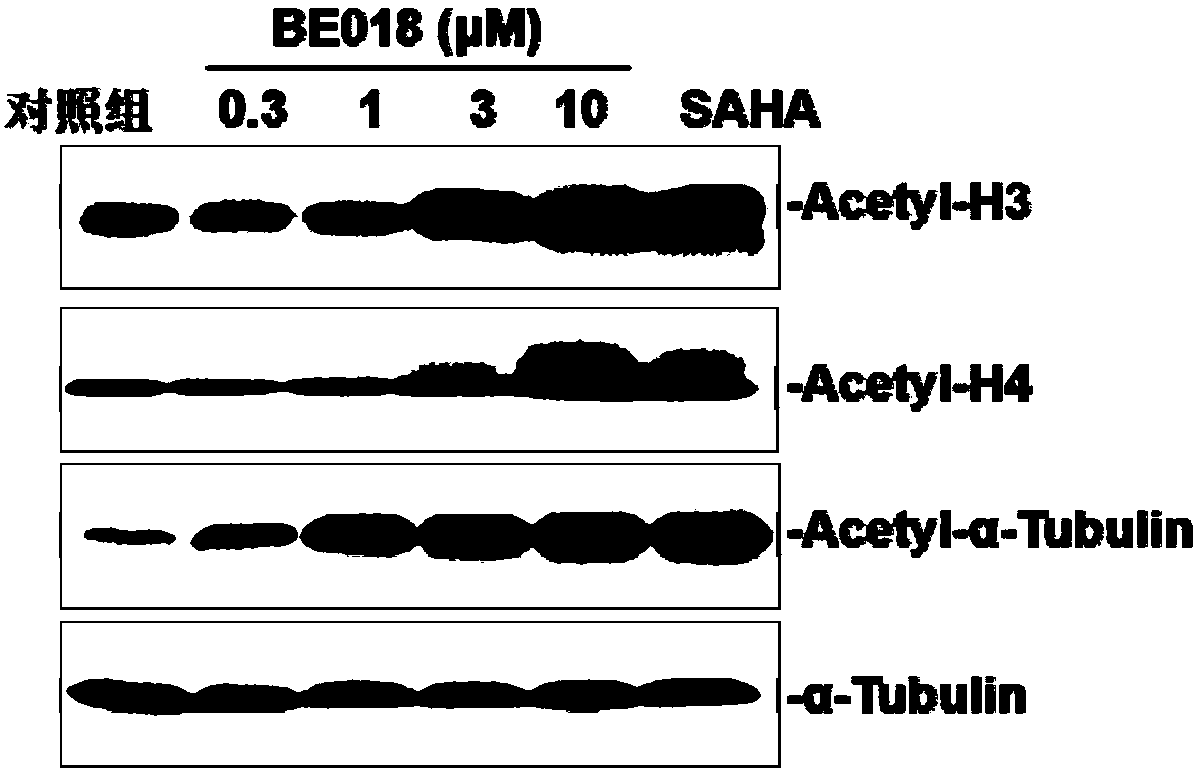
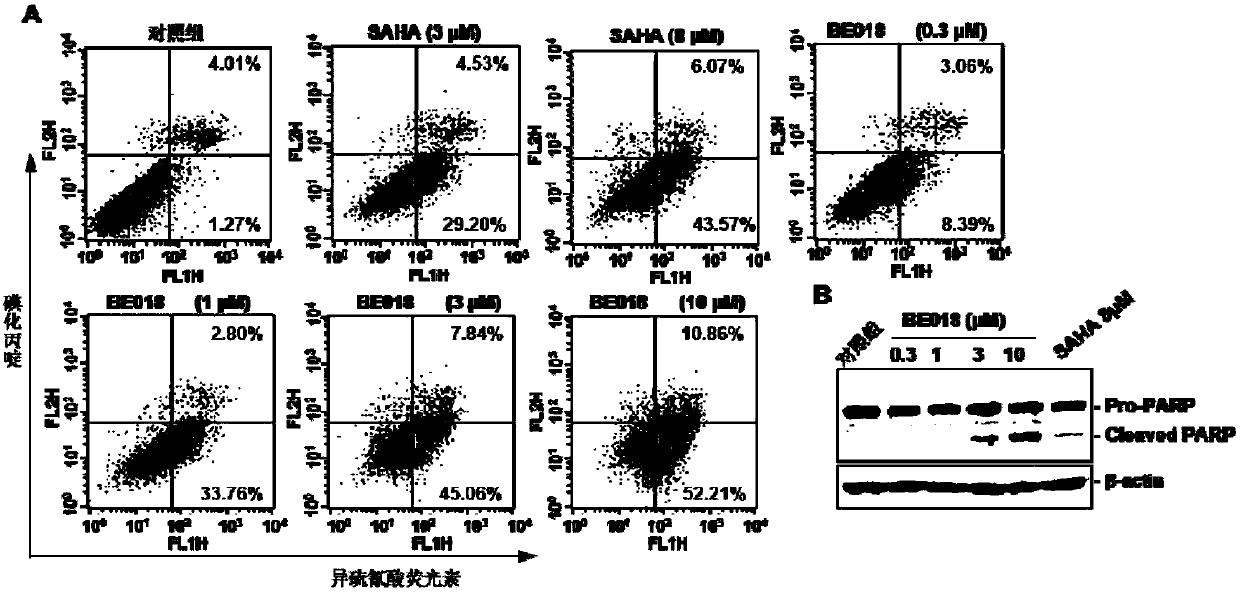
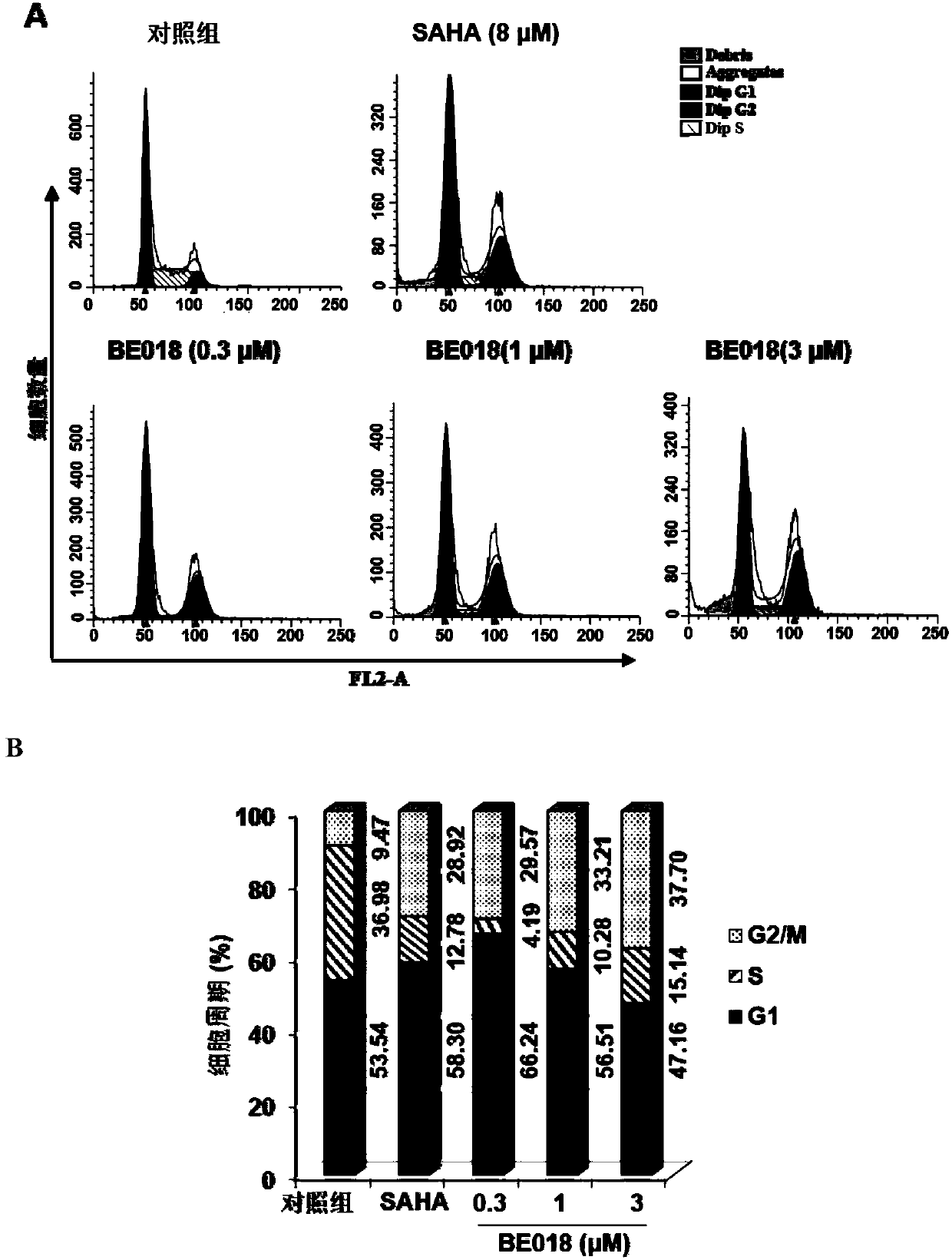
Amide small-molecule organic compound taking indole or indole analogue as parent nucleus structure, application and preparation method of amide small-molecule organic compound
A technology of organic compounds and analogs, applied in the field of drug synthesis, can solve problems such as histone hypoacetylation, chromatin dense and coiled, and tumor suppressor genes cannot be expressed normally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0125] 1 H-NMR was measured with a Bruker 500MHz instrument; MS was measured with a Bruker MicroTOF-Q LCMS instrument, and all were ESI methods unless specified; all solvents were re-distilled before use, and the anhydrous solvents used were all according to the standard method obtained by drying; except for the instructions, all reactions were carried out under the protection of argon and followed by TLC, and the post-treatment was washed with saturated brine and dried with anhydrous magnesium sulfate; the purification of the product was performed using silica gel (200 -300 mesh) column chromatography; the silica gel used, including 200-300 mesh and GF 254 Produced for Qingdao Ocean Chemical Factory or Yantai Yuanbo Silicone Company. Example 1-1, Preparation of Compound (7-(3-((1-adamantyl)formyl))-indolyl)heptanyl hydroxylamide (BE001)
[0126]
[0127] Take 3-indolecarboxylic acid (806mg, 5.0mmol) in dimethylformamide (10ml), cool the reaction system with an ice-water ...
Embodiment 1-2 to 1-9
[0130] Preparation of BE series compounds shown in embodiment 1-2 to 1-9, table 1 (see reference below for specific process)
[0131] Table 1
[0132]
[0133]
Embodiment 1-10
[0134] Example 1-10, Preparation of Compound (7-(3-((2-adamantyl)formyl))-indolyl)heptanyl hydroxylamide (BE010)
[0135]
[0136] Take 3-indolecarboxylic acid (323mg, 2.0mmol), 2-adamantanamine hydrochloride (376mg, 2.0mmol), EDC.HCl (384mg, 2.0mmol), HOBt (271mg, 2.0mmol) in dimethyl form Amide (5ml). The intermediate was obtained after 4h of reaction.
[0137] Take the intermediate (135mg, 0.46mmol) in dimethylformamide (5ml), and slowly add sodium hydride (37mg, 0.92mmol) in an ice-water bath until no bubbles are generated, then add methyl 7-bromoheptanoate ( 123 mg, 0.55 mmol). After 30 min the intermediate ester was obtained.
[0138]Add KOH (2.83g, 50.7mmol) to a methanol (10ml) solution of hydroxylamine hydrochloride (3.33g, 50.7mmol) at 40°C, react for 10min and place the reaction system in an ice bath, and suction filter after 30min to obtain the filtrate. Then the above ester (118mg, 0.27mmol) was added to the filtrate, and KOH (333mg, 5.07mmol) was added to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com