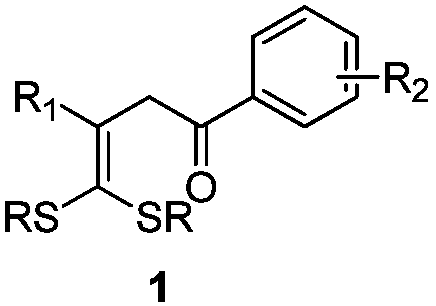
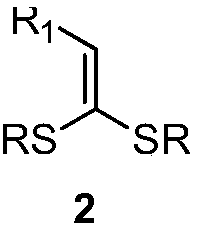
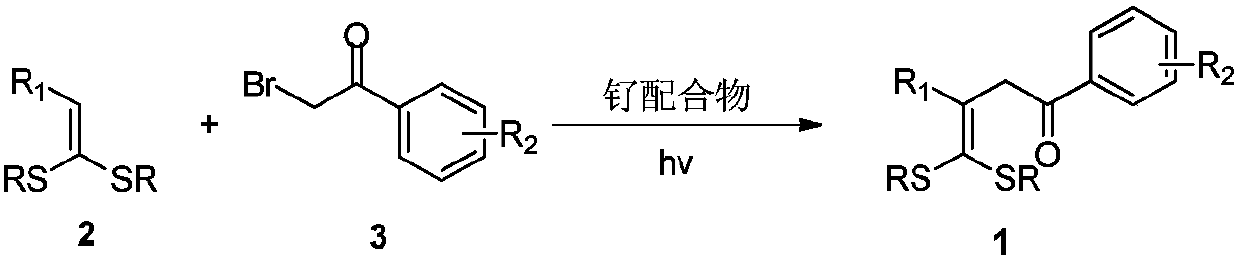
4,4-dialkylthio-1-phenyl-3-buten-1-one derivatives and synthesis method thereof
A synthesis method and technology of dithioketal are applied in the field of 4,4-dialkylthio-1-phenyl-3-butene-1-one derivatives and synthesis, and can solve internal olefin alkylation reaction There are few reports and no reports of alkylation reactions, etc., to achieve the effects of a wide range of substrates, diverse preparations, and high atom economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] The specific process is: weigh dithioketene 2a (67mg, 0.3mmol), Ru(bpy) in the glove box 3 Cl 2 (4mg, 0.006mmol), sodium carbonate (25mg, 0.3mmol), α-bromoacetophenone 3a (119mg, 0.6mmol), was added to a 25mL tube with a branch, and acetonitrile (3mL) was added under nitrogen atmosphere Under the irradiation of 26W white CFL lamp, react at room temperature for 24h. After the reaction was complete, the solvent was removed by rotary evaporation under reduced pressure, followed by column chromatography (petroleum ether (60-90°C) / ethyl acetate: 10:1, v / v) to obtain a light yellow liquid product 1a (72 mg, yield 70 %). The target product was confirmed by NMR spectroscopy.
[0031]
[0032] The specific process is: weigh 1a (68mg, 0.2mmol), NH 2 NH 2 ·H 2 O (120uL, 2.0mmol, 85%) was added to a 25mL sealed tube, 2mL of toluene was added, and the mixture was placed in an oil bath at 120°C for 12h. After the reaction was complete, cool to room temperature, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com