Methylchlorinated fluorocyanopyrethroid compound and preparation method and application thereof
A technology for pyrethroids and compounds, which is applied in the field of pesticides containing cyclopropane carboxylic acid or its derivatives, can solve the problem that it is difficult to meet the requirements of pyrethroids, methyl permethrin has not been applied in mass production, and insecticides are difficult to meet. Low activity and other problems, to achieve the effects of reducing resistance and environmental residues, low production costs, and reduced synthesis difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
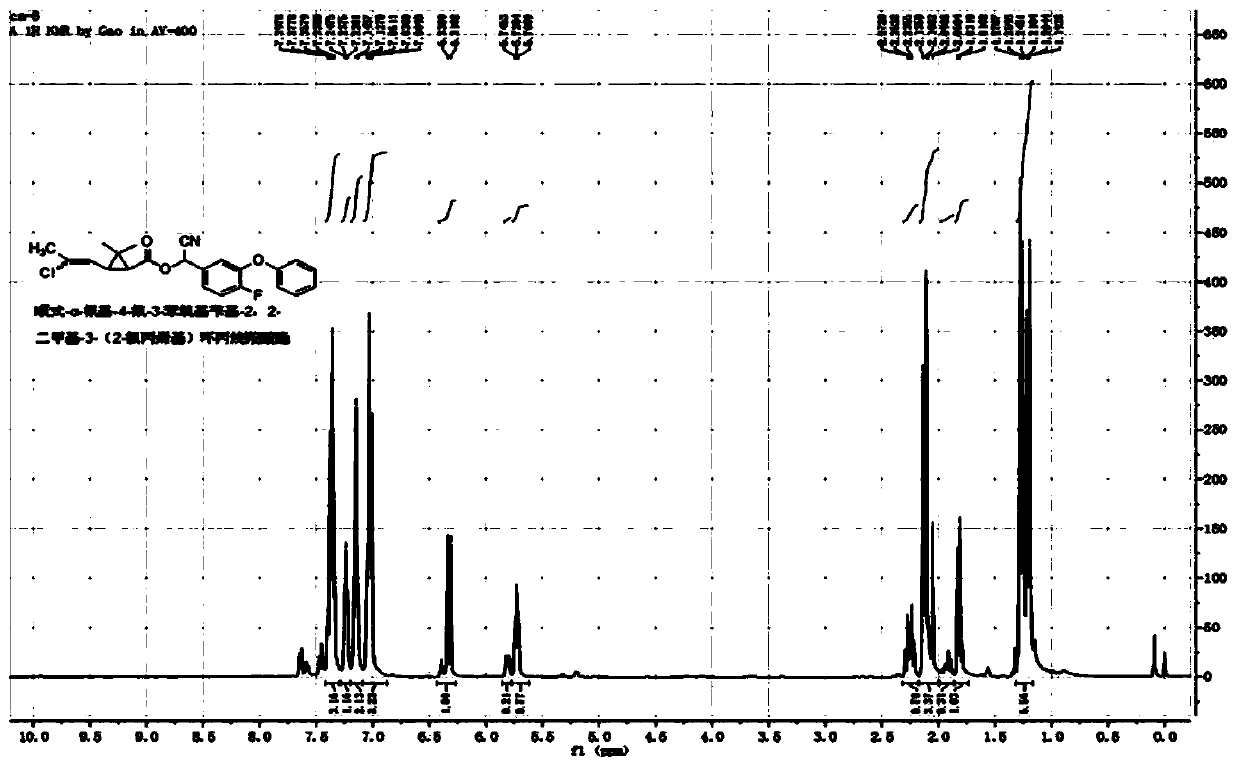
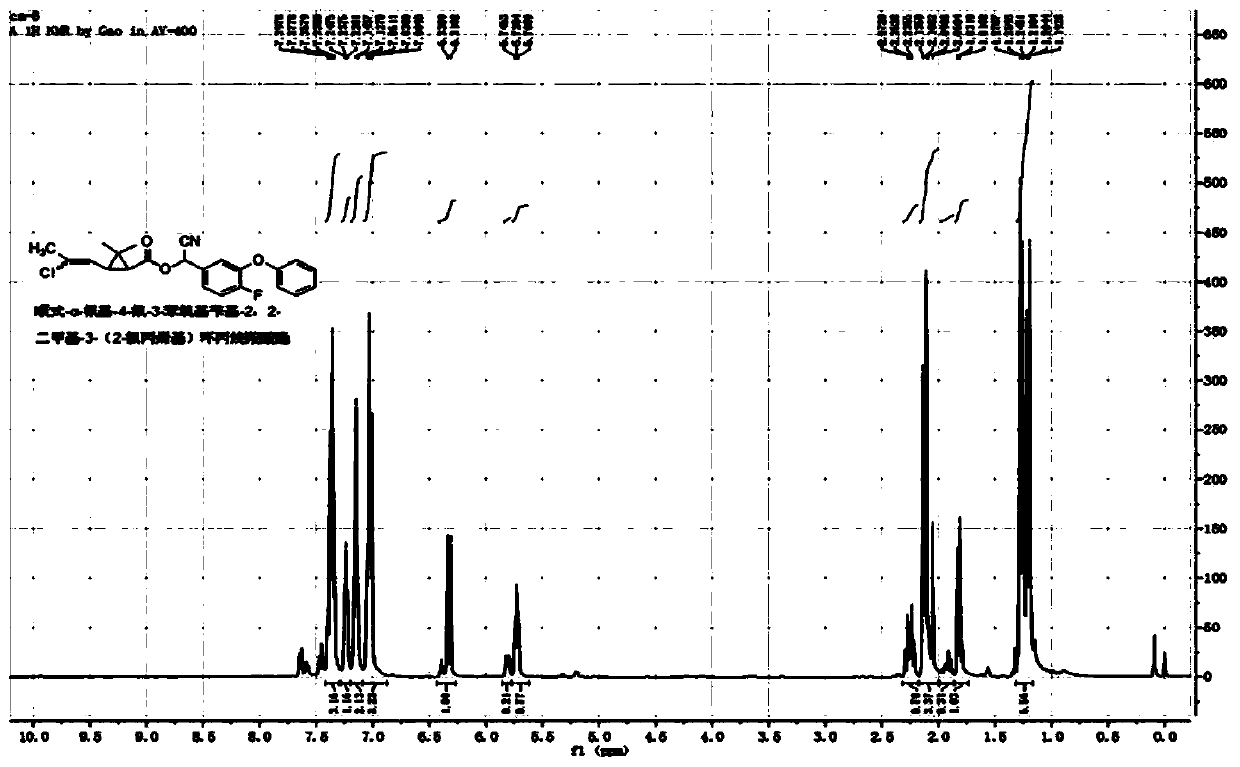
[0046] Example 1: cis / trans-α-cyano-4-fluoro-3-phenoxybenzyl-2,2-dimethyl-3-(E / Z-2-chloropropenyl)cyclopropane Synthesis of Carboxylate
[0047] In a 100mL three-necked flask, add 5.4g (25mmol) of 4-fluoro-3-phenoxybenzaldehyde, 2g of pyridine, 40mL of cyclohexane, and a saturated aqueous solution formed by 1.3g (25mmol) of sodium cyanide, and slowly add cis Formula / trans-2,2-dimethyl-3-(E / Z-2-chloropropenyl)cyclopropanecarboxylic acid chloride 5.2g (25mmol), react at room temperature for 4 hours. The organic phase was washed successively with 20mL of 5% sodium hydroxide solution, 5% hydrochloric acid solution and saturated sodium chloride solution, dried, and after desolvation, a light yellow viscous liquid was obtained. After column chromatography (petroleum ether / ethyl acetate=20 After / 1), 9.4 g of light yellow liquid was obtained, with a yield of 91%. The chemical shifts of the H NMR spectrum are as follows: 1 H NMR (400MHz, CDCl 3 )δ1.13-1.30(m,-CH 3 ,3H),1.52-1.83(...
Embodiment 2
[0048] Example 2: Synthesis of cis-α-cyano-4-fluoro-3-phenoxybenzyl-2,2-dimethyl-3-(2-chloropropenyl)cyclopropanecarboxylate
[0049] In a 100mL three-necked flask, add 5.4g (25mmol) of 4-fluoro-3-phenoxybenzaldehyde, 2.5g of triethylamine, 40mL of toluene, and a saturated aqueous solution formed by 1.8g (37mmol) of sodium cyanide, and slowly add 6 g (29 mmol) of cis-2,2-dimethyl-3-(2-chloropropenyl)cyclopropanecarboxylic acid chloride were reacted at room temperature for 4 hours. The organic phase was washed successively with 20mL of 5% sodium hydroxide solution, 5% hydrochloric acid solution and saturated sodium chloride solution, dried, and after desolvation, a light yellow viscous liquid was obtained. After column chromatography (petroleum ether / ethyl acetate=20 After / 1), 9.8 g of light yellow liquid was obtained, with a yield of 95%. The chemical shifts of the H NMR spectrum are as follows: 1 H NMR (400MHz, CDCl 3 )δ1.19-1.28(m,-CH 3 ,6H),1.81-1.83(m,cyclo-H,1H),2.05...
Embodiment 3
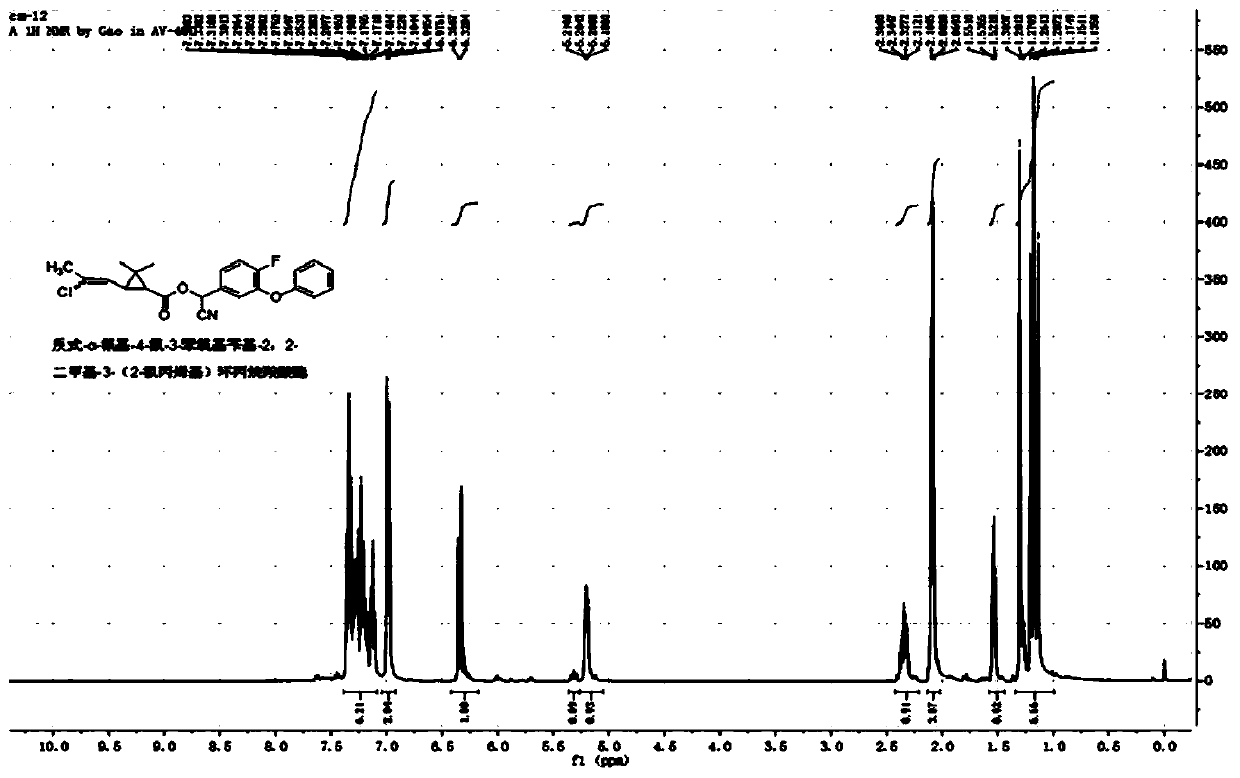
[0050] Example 3: Synthesis of trans-α-cyano-4-fluoro-3-phenoxybenzyl-2,2-dimethyl-3-(2-chloropropenyl)cyclopropanecarboxylate
[0051] Add 6.1 g (25 mmol) of α-cyano-4-fluoro-3-phenoxybenzyl alcohol, 2.5 g of pyridine, and 40 mL of xylene into a 100 mL three-necked flask, and slowly add trans-2,2-dimethyl 5.2 g (25 mmol) of 3-(2-chloropropenyl)cyclopropanecarboxylic acid chloride was reacted at room temperature for 4 hours. Wash successively with 20mL of 5% sodium hydroxide solution, 5% hydrochloric acid solution and saturated sodium chloride solution, dry, and obtain a light yellow viscous liquid after desolvation, through column chromatography (petroleum ether / ethyl acetate=20 / 1 ) to obtain 9.0 g of light yellow liquid with a yield of 87%. The chemical shifts of the H NMR spectrum are as follows: 1 H NMR (400MHz, CDCl 3 ): δ1.13, 1.17, 1.20, 1.30 (4s, -CH 3 ,6H),1.52-1.55(m,cyclo-H,1H),2.07,2.10(2s,-CH 3 ,3H),2.31-2.36(m,cyclo-H,1H),5.18-5.21(m,=CH-,1H),6.33-6.35(m,-CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com