Ophthalmic agent and ophthalmologic drug
A preparation and inhibitor technology, applied in the field of ophthalmic preparations and ophthalmic medicines, can solve problems such as symptomatic therapy of unknown heaviness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0167] Hereinafter, the present invention will be described in more detail by way of examples. The following examples are intended to illustrate the invention, but not to limit it.
[0168] [Viscosity (mPa·s)]
[0169] The ophthalmic preparations produced in Examples and Comparative Examples were subjected to viscosity measurement at 20° C. using an E-type viscometer (“VISCONIC ELD-R”, manufactured by Tokyo Keiki Co., Ltd.).
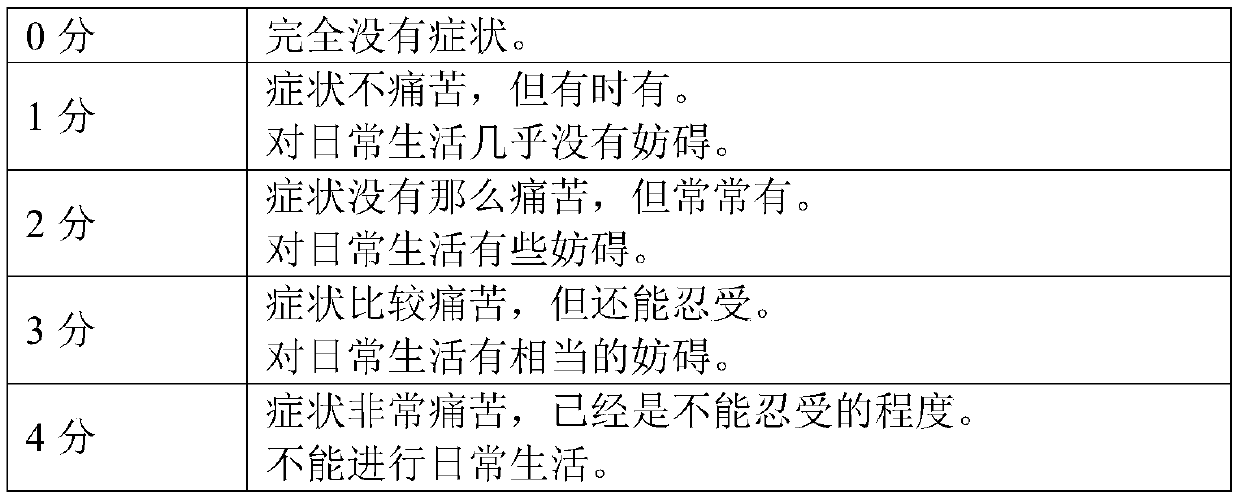
[0170] [Evaluation of subjective symptoms]
[0171] People who have symptoms of dry eyes and belong to the diagnostic criteria of dry eye syndrome (New Ophthalmology 24 (2): 181-184, 2007) are more likely to have "eye gum", "heavy feeling", "eye fatigue (eye fatigue) )", "blurred vision", "congestion", "photophobia", "eye itching" and 7 panelists were tested. Two weeks were set as the pre-observation period, and during the pre-observation period, eye drops (artificial tears "artificial tear eye drops (manufactured by Senju Pharmaceutical Co., Ltd.)") ...
Embodiment 1
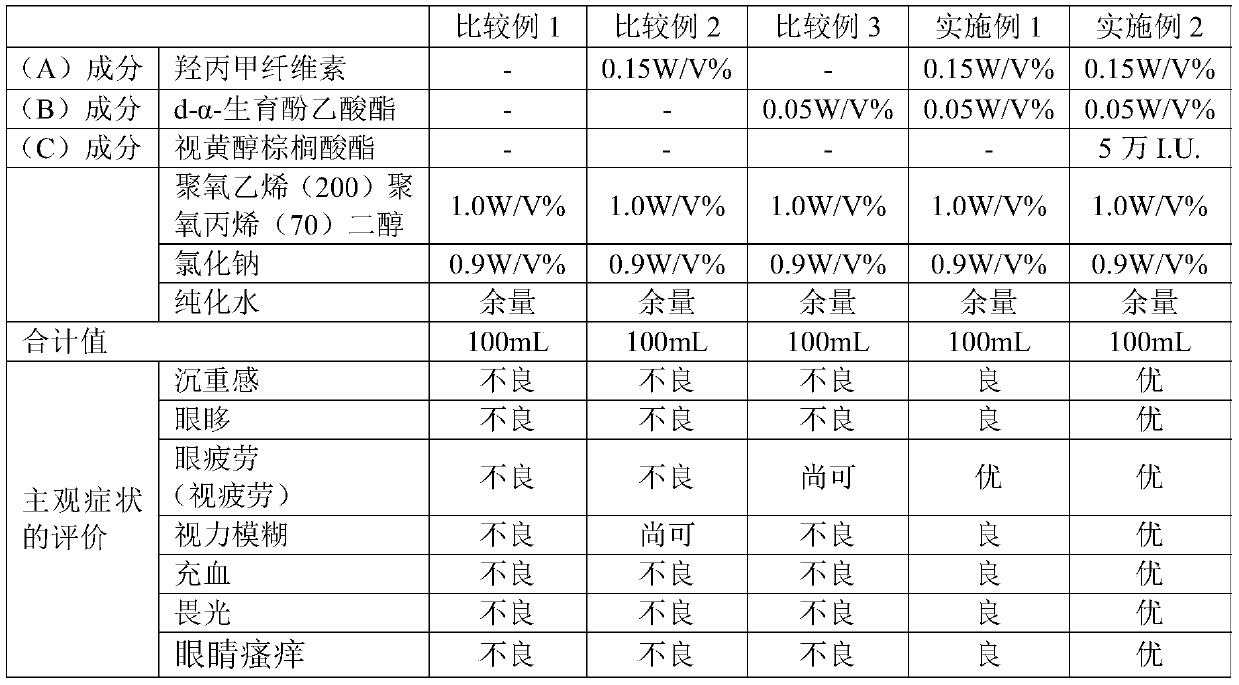
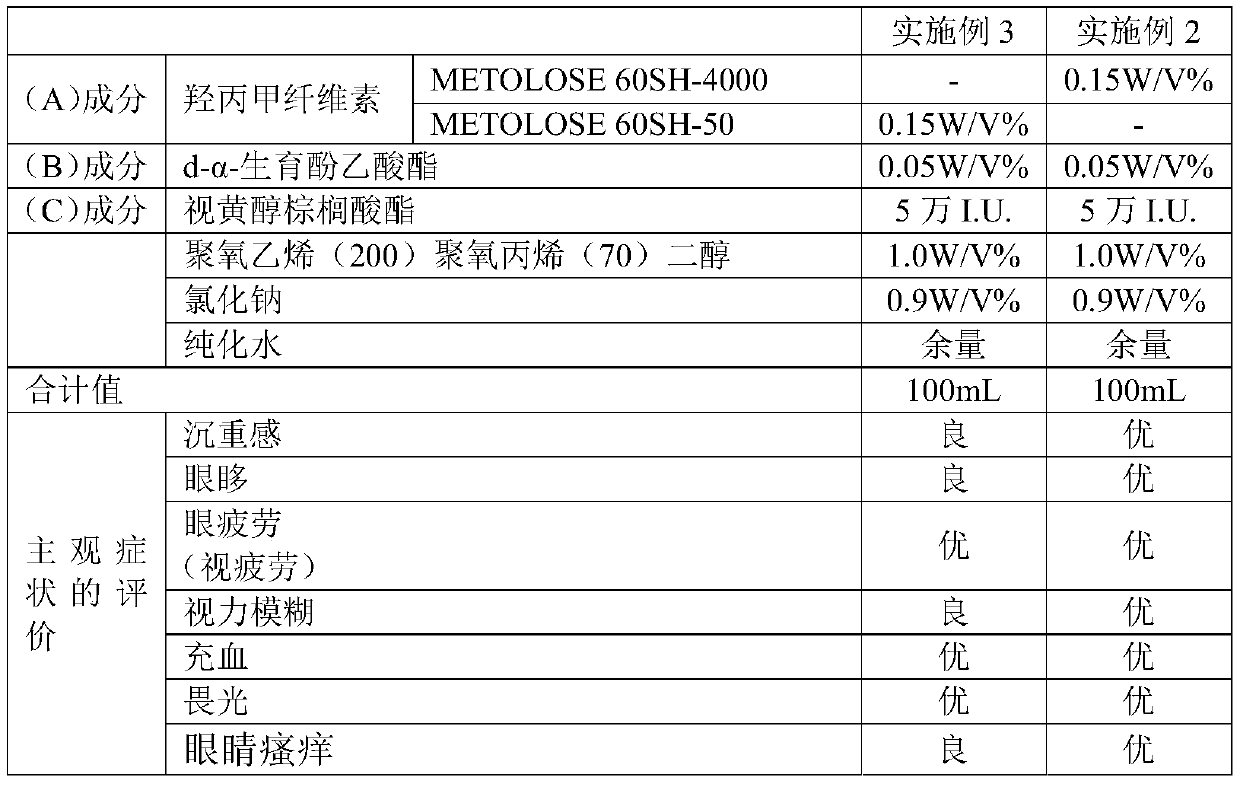
[0188] After dissolving 0.05 g of d-α-tocopheryl acetate (manufactured by Riken Vitamin Co., Ltd., "Riken Acetate α") in purified water through 1.0 g of polyoxyethylene (200) polyoxypropylene (70) diol, Hypromellose 0.15 g (manufactured by Shin-Etsu Chemical Co., Ltd., "METOLOSE 60SH-4000"; weight average molecular weight about 300,000) and sodium chloride 0.9 g were dissolved in purified water to prepare an ophthalmic preparation (test drug) 100mL. The produced ophthalmic preparation had a viscosity of 7 mPa·s at 20°C and a pH of 7.1 at 20°C. Using the produced ophthalmic preparations, evaluation of subjective symptoms was performed. The results are shown in Tables 2 and 4.
Embodiment 2
[0190] 0.05 g of d-α-tocopheryl acetate (manufactured by Riken Vitamin Co., Ltd., "Riken Acetate α") and retinyl palmitate 50,000 I.U. (manufactured by Roche Vitamin Japan Co., Ltd., "palmitic acid Retinol") was dissolved in purified water with 1.0 g of polyoxyethylene (200) polyoxypropylene (70) diol, and 0.15 g of hypromellose (manufactured by Shin-Etsu Chemical Co., Ltd., ) and 0.9 g of sodium chloride were dissolved in purified water to prepare 100 mL of an ophthalmic preparation (test drug). The produced ophthalmic preparation had a viscosity of 7 mPa·s at 20°C and a pH of 7.1 at 20°C. Using the produced ophthalmic preparations, evaluation of subjective symptoms was performed. The results are described in Tables 2-5.
[0191] 【Table 2】
[0192]
[0193] From the results of Comparative Example 1, it can be seen that the ophthalmic preparation containing no active ingredient did not produce the desired efficacy at all. In addition, from the results of Comparative Ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com