Modified T cells and preparation method and application thereof
A technology of cells and cell receptors, applied in the fields of gene editing and tumor immunotherapy, can solve problems such as limited clinical response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
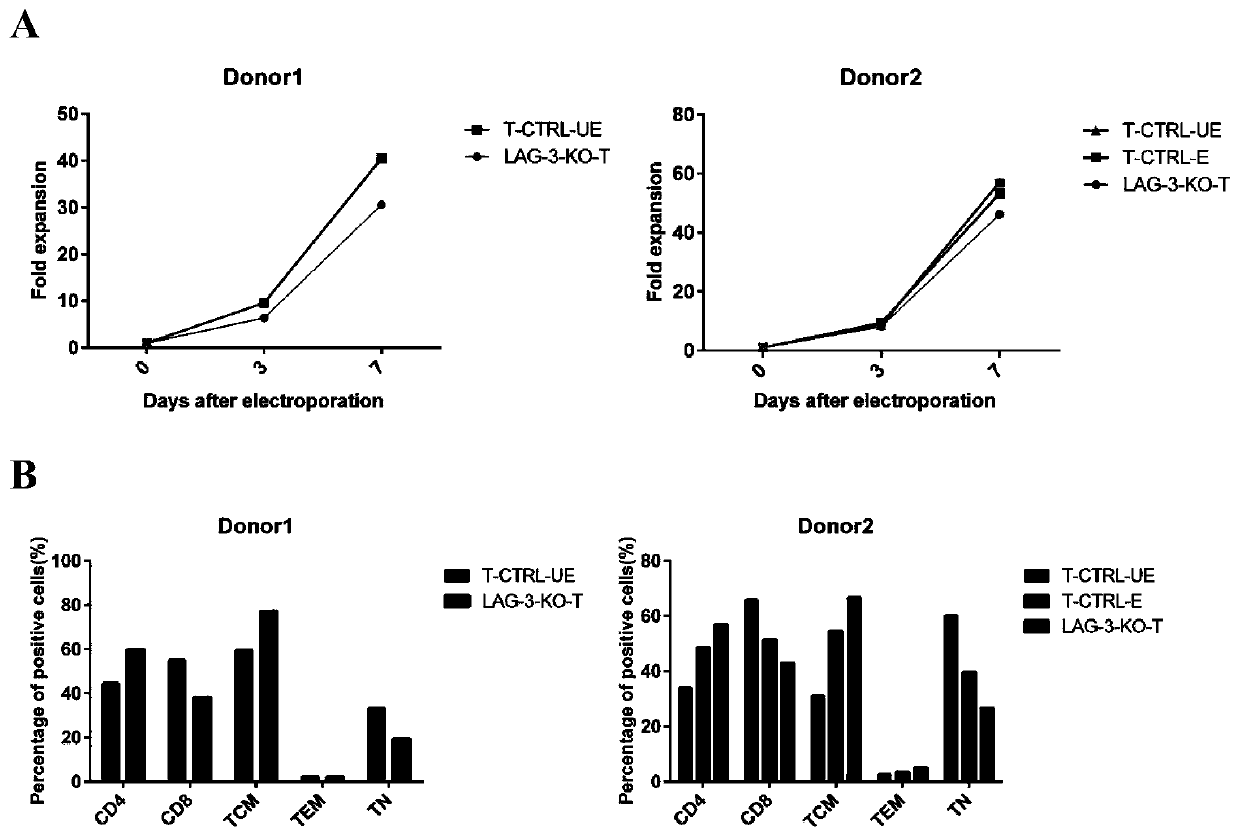
[0213] Example 1. Knockout of the LAG-3 gene in CAR-T cells
[0214] 1. Screen the most effective sgRNA targeting LAG-3 on T cells
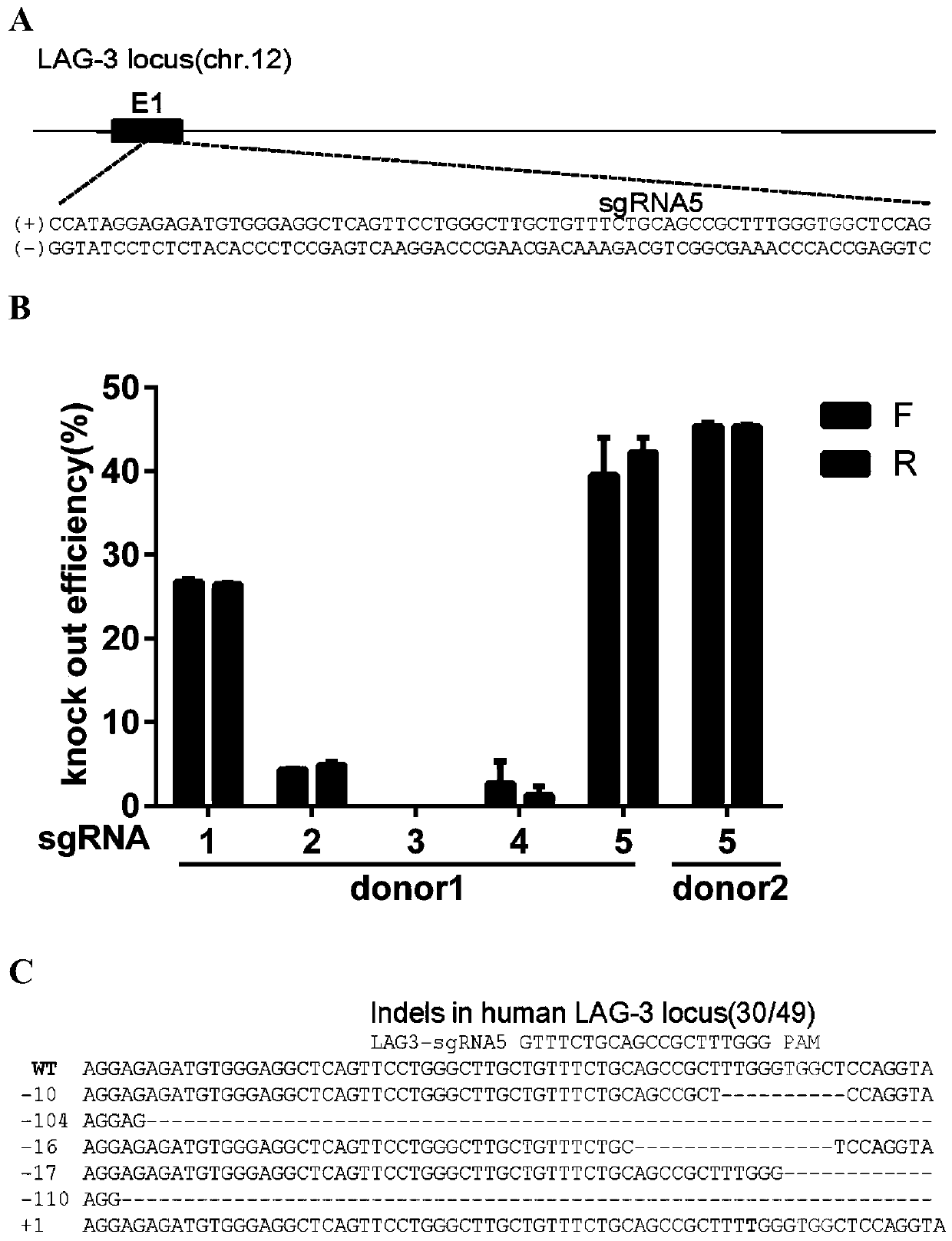
[0215] To abolish LAG-3 expression in T cells, five sgRNAs were designed targeting the first exon of LAG-3. figure 1 A takes sgRNA5 as an example, illustrating the position of the sgRNA in the LAG-3 locus.
[0216] The targeting sequences of the involved sgRNAs are shown in Table 1.
[0217] Table 1. sgRNA targeting LAG-3
[0218] sgRNA
target sequence
SEQ ID NO
sgRNA1
ATGTGGGAGGCTCAGTTCCT
1
sgRNA2
GCTGCAGAAACAGCAAGCCC
2
sgRNA3
TGCTGTTTCTGCAGCCGCTT
3
sgRNA4
GCTGTTTCTGCAGCCGCTTT
4
sgRNA5
GTTTCTGCAGCCGCTTTGGG
5
[0219] Cas9 protein (3 μg) was complexed with in vitro transcribed sgRNA (3 μg), and then electroporated into primary CD3 + in T cells. The gene editing efficiency of each sgRNA was quantified by TIDE analysis, and the most effective sgRNA was ...
Embodiment 2
[0236] Example 2. Knocking out the CTLA-4 gene in CAR-T cells
[0237] 1. Screening sgRNA targeting CTLA-4
[0238] Design five kinds of sgRNA targeting exon 1 coding region of CTLA-4 locus, target sequence such as SEQ ID NO:6-10 (Table 2), such as Figure 6 In A, the targeting sequence of sgRNA1 is in green, and the PAM sequence is in blue.
[0239] Table 2. sgRNA targeting CTLA-4
[0240]
[0241]
[0242] Cas9 protein (3 μg) and in vitro transcribed sgRNA (3 μg) were complexed to form Cas9-sgRNA ribonucleoprotein (RNP), and then electroporated into primary 1×10 6 CD3+ T cells (3 days after activation).
[0243] Knockdown efficiency using each sgRNA was quantified by TIDE analysis, and indel frequency in CTLA-4 was analyzed by sequencing. The result is as Figure 6 As shown in B, sgRNA1 has the highest knockout efficiency, and gene editing with sgRNA1 in another donor (donor 2) also obtained a significant knockout effect.
[0244] PCR products from each sample we...
Embodiment 3
[0262] Example 3, knockout of the Foxp3 gene in CAR-T cells
[0263] 1. Screening of sgRNA targeting Foxp3
[0264] Design six kinds of sgRNA targeting Foxp3 gene locus exon 2 coding region, the sequence is as SEQ ID NO:11-15 (Table 3), such as Figure 11 In A, the targeting sequence of sgRNA3 is in green, and the PAM sequence is in blue.
[0265] Table 3. sgRNA targeting Foxp3
[0266] sgRNA
Foxp3 target sequence
SEQ ID NO
sgRNA1
GGGCCGAGATCTTCGAGGCG
11
sgRNA2
TCGAAGATCTCGGCCCTGGA
12
sgRNA3
GCAGCTGCGATGGTGGCATG
13
sgRNA4
AGGGCCGAGATCTTCGAGGC
14
sgRNA5
GGCCCTGGAAGGTTCCCCCT
15
sgRNA6
TTTGGGTGCAGCCTCCAGC
16
[0267] Cas9 protein (3 μg) and in vitro transcribed sgRNA (3 μg) were complexed to form Cas9-sgRNA ribonucleoprotein (RNP), and then electroporated into primary 1×106 In CD3+ T cells (3 days after activation).
[0268] The knockout efficiency of each sgRNA was quanti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com