Feooh/ni, a micron flower-shaped high-performance full-splitting bifunctional electrocatalyst for water 3 the s 2 preparation method
An electrocatalyst and microflora technology, which is applied to electrodes, electrolysis components, electrolysis processes, etc., can solve the problems of inability to exhibit electrocatalytic performance, high price, and application limitations, and achieve good electrocatalytic total water splitting performance and preparation method. Simple, low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Step 1: Cut the commercially available nickel foam into long strips with a size of 2cm*1cm, wash it in 3M HCl solution for 15 minutes, then use ethanol and acetone to ultrasonically clean it for 20 minutes, and finally use ultrapure water Ultrasound for 10 minutes to clean it, then dry it for later use;
[0039] Step 2: Add 0.8724g (3mmol) of nickel nitrate, 0.2283g (3mmol) of thiourea, and 0.1g (0.3mmol) of hexadecyltrimethylammonium bromide into 25mL of ethanol, and stir until it is completely dissolved. Pour the solution into a 30mL hydrothermal kettle.
[0040] Step 3: Put the cleaned foam nickel (NF) into the solution prepared in step 2 and immerse it in the oven at 150°C for 10 hours. After cooling down to room temperature naturally, take it out and rinse it with deionized water and ethanol, and put it in 80 Dry in an oven at ℃ for 3 hours to obtain Ni grown in situ on nickel foam 3 S 2 Material;
[0041] Step 4: Dissolve weighed 0.606g of ferric nitrate in a ...
Embodiment 2
[0044] Other steps are with embodiment 1, and difference is that the nickel nitrate in step 2 is changed into 0.4362g by 0.8724g.
Embodiment 3
[0046] Other steps are with embodiment 1, and difference is that the nickel nitrate in step 2 is changed into 0.2908g by 0.8724g.
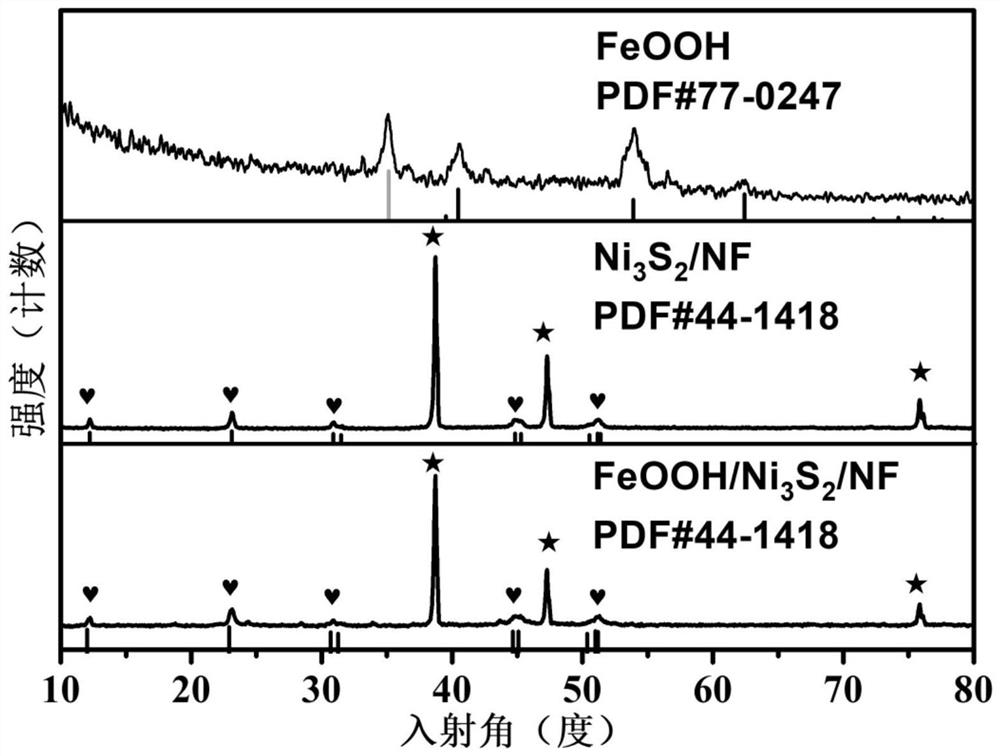
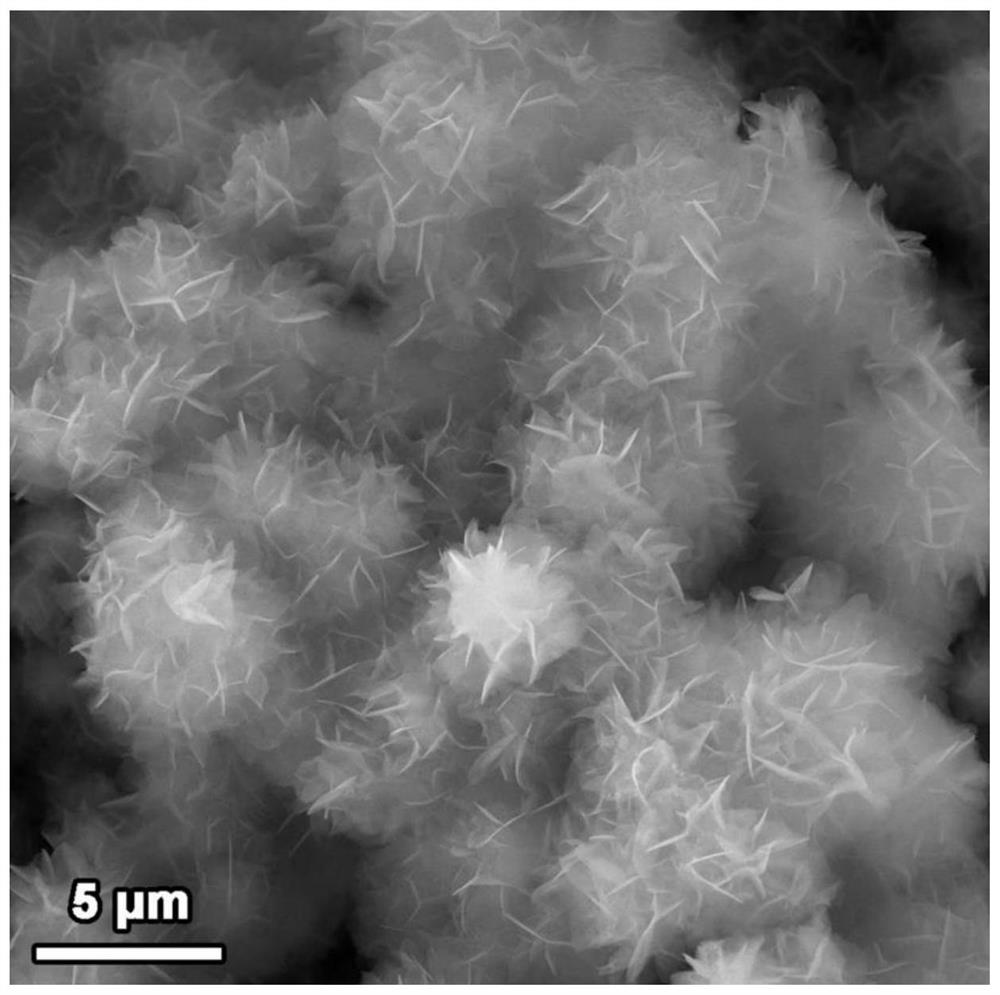
[0047] Test results: Different Ni was prepared by changing the amount of nickel nitrate 3 S 2 Loaded FeOOH / Ni 3 S 2 / NF composite catalyst, and carried out X-ray diffraction, scanning electron microscopy, transmission electron microscopy, linear voltammetry scanning test, electrochemical AC impedance test, stability test, the test results are as follows Figure 1-15 shown.
[0048] figure 1 Be Ni:S=1:2 among the embodiment 5, in Fe(NO 3 ) 3 FeOOH / Ni prepared by deposition in electrolyte for 200s 3 S 2 / NF composite catalyst with FeOOH nanosheets and Ni 3 S 2 In the X-ray diffraction pattern of / NF, FeOOH nanosheets have several strong diffraction peaks at 34.1 degrees, 42.2 degrees, 53.6 degrees, and 63.5 degrees, corresponding to the standard PDF card PDF#77-0247. Ni 3 S 2 / NF samples have several strong diffraction peaks at 21.7 deg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com