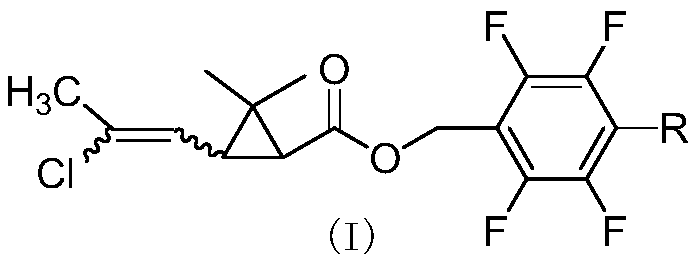
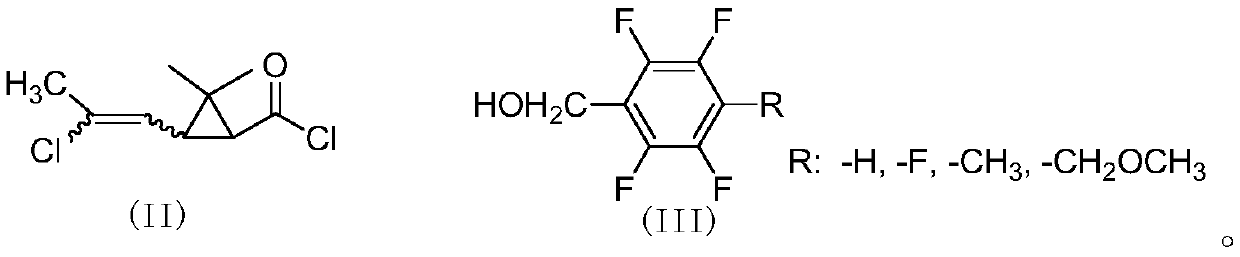
Methyl cloro chrysanthemic acid polyfluoro benzyl alcohol pyrethroid compound as well as preparation method and application thereof
A technology of permethrin and permethrin, which is applied in the preparation of compounds, permethrin compound, the application field of the above-mentioned compounds, can solve the problems that the activity of the compounds needs to be improved, Achieve the effects of reducing resistance and environmental residue problems, high killing activity, and reducing the complexity of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of cis-2,3,5,6-tetrafluorobenzyl 2,2-dimethyl-3-(E / Z-2-chloropropenyl)cyclopropanecarboxylate
[0058] Add 4.5g (25mmol) of 2,3,5,6-tetrafluorobenzyl alcohol, 2g of pyridine, and 40mL of cyclohexane into a 100mL three-necked flask, and slowly add cis-2,2-dimethyl-3-( E / Z-2-chloropropenyl)cyclopropanecarboxylic acid chloride 5.2g (25mmol), react at room temperature for 4 hours. The organic phase was washed successively with 20mL of 5% sodium hydroxide solution, 5% hydrochloric acid solution and saturated sodium chloride solution, dried, and after desolvation, a light yellow viscous liquid was obtained, which was subjected to column chromatography (petroleum ether / ethyl acetate=20 After / 1), 8.0 g of light yellow liquid was obtained, with a yield of 91%. 1 H NMR (400MHz, CDCl 3 )δ1.23,1.24,1.25,1.27(4s,-CH 3 ,6H),1.77(2d,cyclo-H,J 1 =8.4Hz,J 2 =5.8Hz,1H),1.87(t,cyclo-H,J=8.5Hz,0.3H),2.10,2.13(2s,-CH 3 ,3H),2.18(t,cyclo-H,J=8.4Hz,0.7H),5.18-5.25(m,-OCH 2 -...
Embodiment 2
[0061] Synthesis of cis-2,3,4,5,6-pentafluorobenzyl 2,2-dimethyl-3-(E / Z-2-chloropropenyl)cyclopropanecarboxylate
[0062] Add 4.95g (25mmol) of 2,3,4,5,6-pentafluorobenzyl alcohol, 2.5g of triethylamine, and 40mL of toluene into a 100mL three-necked flask, slowly add cis-2,2-dimethyl- 6 g (29 mmol) of 3-(E / Z-2-chloropropenyl)cyclopropanecarboxylic acid chloride was reacted at room temperature for 4 hours. The organic phase was washed successively with 20mL of 5% sodium hydroxide solution, 5% hydrochloric acid solution and saturated sodium chloride solution, dried, and after desolvation, a light yellow viscous liquid was obtained, which was subjected to column chromatography (petroleum ether / ethyl acetate=20 After / 1), 9.0 g of light yellow liquid was obtained, with a yield of 96%. 1 H NMR (400MHz, CDCl 3 )δ0.98-1.24(m,-CH 3 ,6H),1.62-1.75(m,cyclo-H,1H),1.85-2.18(m,-CH 3 and cyclo-H,4H), 5.11(d,=CH-,0.7H),5.65-5.85(d,=CH-,0.3H,J=8.6Hz); HRMS cacld for C 23 h 22 NO 3 FCl ...
Embodiment 3
[0064] Synthesis of cis-4-methyl-2,3,5,6-tetrafluorobenzyl 2,2-dimethyl-3-(E / Z-2-chloropropenyl)cyclopropanecarboxylate
[0065] Add 4.85g (25mmol) of 4-methyl-2,3,5,6-tetrafluorobenzyl alcohol, 2.5g of pyridine, and 40mL of xylene into a 100mL three-necked flask, and slowly add cis-2,2-dimethyl 5.2 g (25 mmol) of 3-(2-chloropropenyl)cyclopropanecarboxylic acid chloride was reacted at room temperature for 4 hours. Wash successively with 20mL of 5% sodium hydroxide solution, 5% hydrochloric acid solution and saturated sodium chloride solution, dry, and obtain light yellow viscous liquid after desolvation, through column chromatography (petroleum ether / ethyl acetate=20 / 1 ) to obtain 8.5 g of light yellow liquid with a yield of 93%. 1 H NMR (400MHz, CDCl 3 )δ1.22,1.23,1.25,1.26(4s,-CH 3 ,6H),1.73-1.77(m,cyclo-H,1H),1.85(t,cyclo-H,J=8.8Hz,0.3H),2.10-2.19(m,-CH 3 ,cyclo-H,3.7H),5.14-5.22(m,-OCH 2 -,2H),5.80(d,=CH-,J=8.2Hz,0.7H),5.86(d,=CH-,J=8.5Hz,0.3H); HRMScacld for C 17 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com