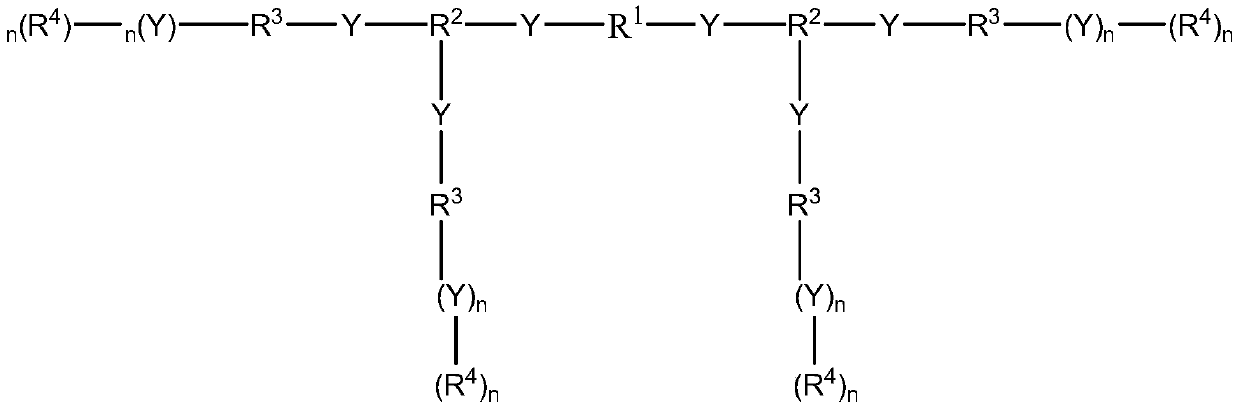
Medical polyurethane adhesive and preparation method thereof
A technology of polyurethane adhesives and polycondensation products, applied in the direction of polyurea/polyurethane adhesives, adhesive types, adhesives, etc., can solve problems such as the risk of carrying viruses, poor adhesion, and difficulty in mass production, and achieve Good formulation adjustability and adaptability, improved biological applicability, and the effect of promoting good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] (1) Add 0.24g of PEG with a molecular weight of 400 into a 500mL four-necked flask equipped with a stirrer and a thermometer, and carry out stirring and dehydration treatment at 110°C under a vacuum of 0.1MPa, with a stirring speed of 1200r / min for a time of 3h, to obtain dehydrated polyether polyol. The maltodextrin with a DB value of 10 was placed in a vacuum of 0.08MPa and dried at 60°C for 24h.
[0071] (2) Cool down the dehydrated polyether polyol in step (1) to 70°C, then add 0.62g of triethylamine, 30.0g of dimethyl sulfoxide, and 68.91g of IPDI while blowing nitrogen, and stir the reaction at 70°C for 1.5 h, the stirring speed is 700r / min, and the end-NCO polyurethane prepolymer is prepared. During this period, according to the change of the viscosity, the speed of the stirrer was adjusted at any time and an appropriate amount of dimethyl sulfoxide was added.
[0072] (3) Add dimethyl sulfoxide to the end-NCO prepolymer obtained in step (2) to adjust the visco...
Embodiment 2
[0077] (1) Add 0.24g of PEG with a molecular weight of 400 into a 500mL four-neck flask equipped with a stirrer and a thermometer, and carry out stirring and dehydration treatment at 100°C under a vacuum of 0.1MPa, with a stirring speed of 1200r / min for a time of 3h, to obtain dehydrated polyether polyol. The maltodextrin with a DB value of 15 was placed in a vacuum of 0.1 MPa and dried at 70° C. for 48 hours.
[0078] (2) Cool down the dehydrated polyether polyol in step (1) to 75°C, then add 0.32g triethylamine and 0.30g triethanolamine, 50.0g N,N-dimethylformamide, 68.91g IPDI, stirred and reacted at 75° C. for 1 h, and the stirring speed was 800 r / min, to obtain a terminal-NCO polyurethane prepolymer. During this period, according to the change of viscosity, adjust the speed of the stirrer at any time and add an appropriate amount of N,N-dimethylformamide.
[0079] (3) Add N,N-dimethylformamide to the terminal-NCO prepolymer obtained in step (2) to adjust the viscosity, th...
Embodiment 3
[0084] (1) Add 0.24g of PEG with a molecular weight of 400 into a 500mL four-necked flask equipped with a stirrer and a thermometer, and carry out stirring and dehydration treatment at 105°C under a vacuum of 0.05MPa, with a stirring speed of 1000r / min for a time of 3h, to obtain dehydrated polyether polyol. The amylose was placed in a vacuum of 0.08MPa and dried at 60°C for 48h.
[0085] (2) Lower the temperature of the dehydrated polyether polyol in step (1) to 65°C, then add 0.66g of diethylenetriamine, 30.0g of tetrahydrofuran, and 77.58g of MDI while blowing nitrogen, and stir the reaction at 65°C for 1.5h, then stir The speed is 600r / min, and the end-NCO polyurethane prepolymer is prepared. During this period, according to the change of the viscosity, the speed of the stirrer was adjusted at any time and an appropriate amount of tetrahydrofuran was added.
[0086] (3) Add tetrahydrofuran to the terminal-NCO prepolymer obtained in step (2) to adjust the viscosity, then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Shear strength | aaaaa | aaaaa |
| Shear strength | aaaaa | aaaaa |
| Shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com