A kind of anti-his tag heavy chain antibody and application thereof
A heavy-chain antibody and single-domain heavy-chain antibody technology, applied in the field of molecular immunology, can solve the problems of cumbersome monoclonal antibody development and production process, low specificity and instability of polyclonal antibodies, and achieve excellent genetic resources and The effect of antibody resources, strong affinity, and reliable sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
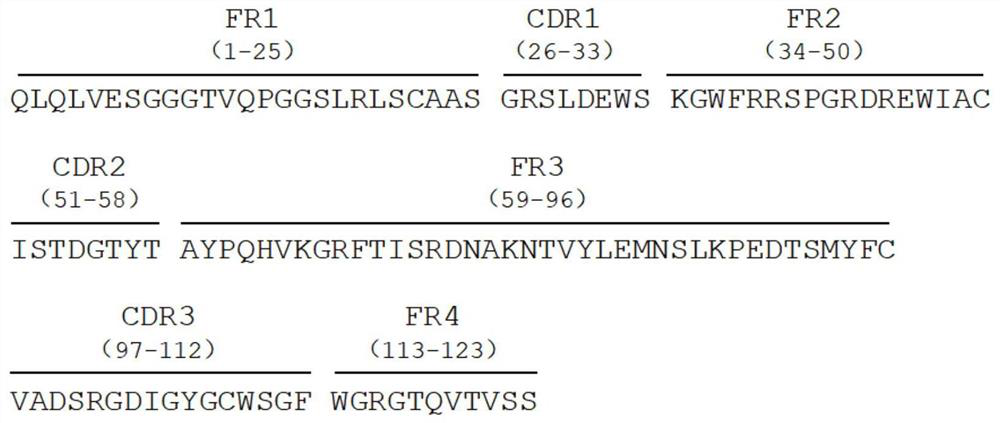
[0027] Example 1 Panning of Anti-His Tag Nanobodies
[0028] The single domain heavy chain antibody targeting His tag was panned from the single domain heavy chain antibody phage display library by solid phase affinity panning. Dilute the His-tagged protein to 30-100 μg / μL with 1×PBS solution, coat it on an ELISA plate, add 100 μL to each well, and coat overnight at 4°C; suck out the coating solution, wash the plate 3 times with PBST, and wash each well 3 times. Add 300 μL of 4% skimmed milk, block at 37°C for 2 hours; wash the plate with PBST three times, add the phage display library (containing about 1×10 12 CFU), incubated at 37°C for 1 h; aspirate the unbound phage, wash the plate 5 times with PBST (increase the number of washes for each round, and the number of washes for each round is shown in Table 1), wash the plate with PBST for 3 times, and then add 100 μL wash Remove the liquid (glycine-hydrochloric acid solution, pH 2.2) to elute the phage adsorbed in the plate w...
Embodiment 2
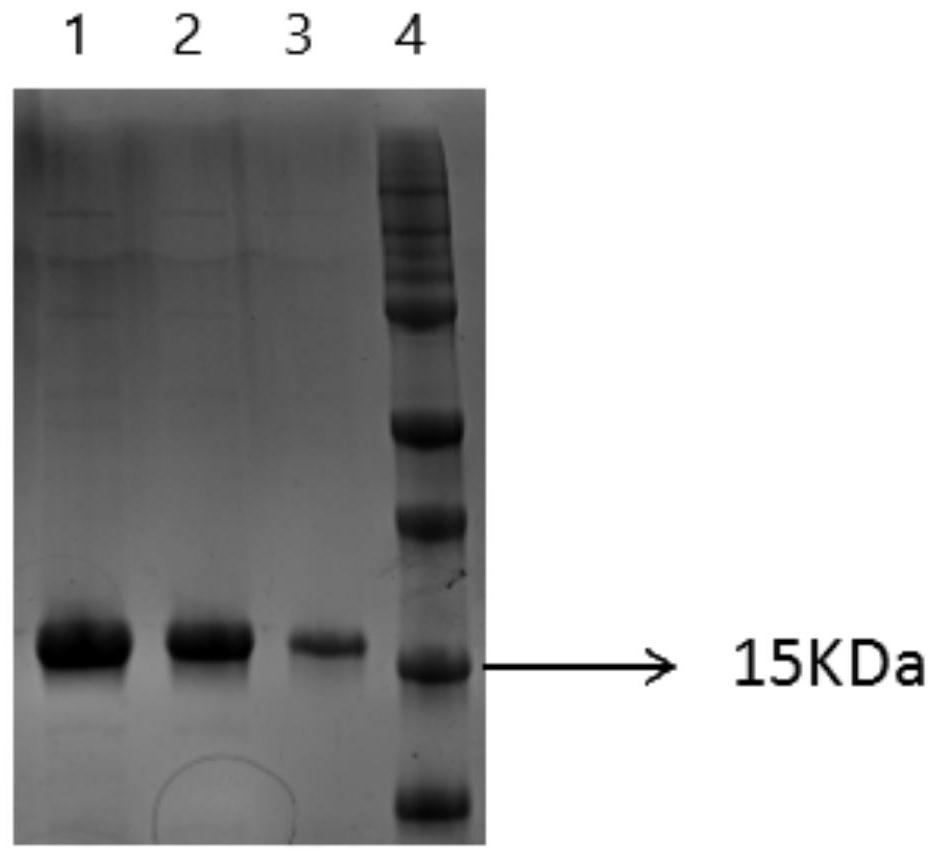
[0037] Example 2 Expression and purification of anti-His tag nanobody
[0038] The gene of the nanobody obtained in Example 1 was cloned into the expression vector pET-25b (cloned by Qingke Biological Co., Ltd.), and an anti-His tag nanobody expression plasmid was constructed. The constructed expression plasmid was transformed into Escherichia coli BL21, and a single clone was picked for induced expression. Inoculate the single clone into 1L LB liquid medium (containing 100 μg / mL ampicillin) for culture, shake culture at 37°C, 220rmp / min until the OD600 of the bacterial solution reaches 0.5, add IPTG with a final concentration of 0.1mM, and grow at 16°C, 80rmp / min min overnight induction culture. After the culture was completed, the cells were collected by centrifugation, resuspended in 50 mL PBS solution, and then ultrasonically disrupted on ice at 200w for 3 seconds with an interval of 4 seconds for a total of 30 minutes. The supernatant was collected by centrifugation at 8...
Embodiment 3
[0040] Example 3 Affinity Determination of Anti-His Tag Nanobody
[0041] Use Octet @ The RED96 intermolecular interaction detection system (ForteBio Company) was used to measure the affinity of the Nanobodies prepared in Example 2. Octet @ RED96 intermolecular interaction detection system is based on biofilm laminography interferometry (BLI) technology, which can measure the interaction between proteins and biomolecules with only a small amount of sample and without labeling.
[0042] The His-tagged protein was diluted to 10 μg / mL, and the nanobody obtained in Example 2 was diluted to 50 μg / mL. The diluent used was PBS+0.1% Tween 20+0.1% BSA, and the samples were loaded according to Table 3. Affinity detection chart see Figure 4 .
[0043] Table 3 Affinity detection sample loading table
[0044]
[0045] Equilibrium dissociation constant (affinity) K D (M)=k dis (1 / s) / k on (1 / Ms)
[0046] It was detected that:
[0047] k dis (1 / s) = 0.009213;
[0048] k on (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com