Posaconazole hydrogel patch and preparing method thereof
A posaconazole hydrogel patch, posaconazole technology, applied in antifungal agents, pharmaceutical formulations, medical preparations with non-active ingredients, etc., can solve the problems of monotonous dosage forms, difficult transportation, and difficult storage , to achieve the effect of preventing infection and preventing external infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
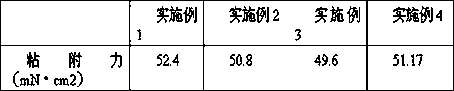
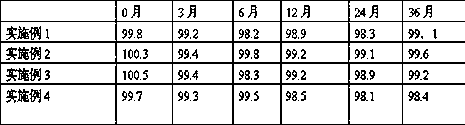
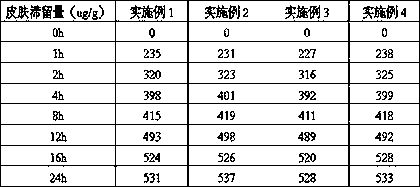
Embodiment 1
[0037] Example 1 Posaconazole gel patch
[0038] Posaconazole 10.0g
[0039] Carbomer-940 20.0g
[0040] Concentrated hydrochloric acid 2.0ml
[0041] Absolute ethanol 50ml
[0042] PEG-400 100.0g
[0043] Hydroxypropyl-β-cyclodextrin 300.0g
[0044] Glycerin 50.0g
[0045] Methylparaben 0.5g
[0046] Ethylparaben 0.5g
[0047] Appropriate amount of triethanolamine
[0048] 1) The preparation method is: slowly disperse the prescribed amount of carbomer into an appropriate amount of distilled water, swell, and leave overnight
[0049] 2) Add an appropriate amount of absolute ethanol and concentrated hydrochloric acid to the prescribed amount of posaconazole, and fully dissolve it. Take the prescribed amount of hydroxypropyl-β-cyclodextrin, add an appropriate amount of distilled water, dissolve it in a hot water bath, slowly add it to the medicinal solution, stir, and inclusion complex at 35°C for 24 hours to obtain a posaconazole inclusion complex solution.
[0050] 3...
Embodiment 2
[0053] Example 2 Posaconazole gel patch
[0054] Posaconazole 15.0g
[0055] Carbomer-940 25.0g
[0056] Concentrated hydrochloric acid 2.3ml
[0057] Absolute ethanol 60ml
[0058] PEG-400 150.0g
[0059] Hydroxypropyl-β-cyclodextrin 360.0g
[0060] Glycerin 55.0g
[0061] Methylparaben 0.55g
[0062] Appropriate amount of triethanolamine
[0063] 1) Slowly disperse the prescribed amount of carbomer into an appropriate amount of distilled water, swell, overnight
[0064] 2) Add an appropriate amount of absolute ethanol and concentrated hydrochloric acid to the prescribed amount of posaconazole, and fully dissolve it. Take the prescribed amount of hydroxypropyl-β-cyclodextrin, add an appropriate amount of distilled water, dissolve it in a hot water bath, slowly add it to the medicinal solution, stir, and inclusion complex at 35°C for 24 hours to obtain a posaconazole inclusion complex solution.
[0065] 3) Slowly add glycerin, PEG-400, methylparaben and ethylparaben int...
Embodiment 3
[0068] Example 3 Posaconazole gel patch
[0069] Posaconazole 15.0g
[0070] Carbomer-940 25.0g
[0071] Concentrated hydrochloric acid 2.3ml
[0072] Absolute ethanol 60ml
[0073] PEG-400 150.0g
[0074] Sulfobutyl-β-cyclodextrin 360.0g
[0075] Glycerin 55.0g
[0076] Methylparaben 0.55g
[0077] Ethylparaben 0.55g
[0078] Appropriate amount of triethanolamine
[0079] 1) Slowly disperse the prescribed amount of carbomer into an appropriate amount of distilled water, swell, overnight
[0080] 2) Add an appropriate amount of absolute ethanol and concentrated hydrochloric acid to the prescribed amount of posaconazole, and fully dissolve it. Take the prescribed amount of sulfobutyl-β-cyclodextrin, add an appropriate amount of distilled water, dissolve it in a hot water bath, slowly add it to the medicinal solution, stir, and inclusion complex at 35°C for 24 hours to obtain a posaconazole inclusion complex solution.
[0081] 3) Slowly add glycerin, PEG-400, methylpar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com