Organic small-molecule efficient room-temperature phosphorescent material on basis of aryl imide, and preparation and application of organic small-molecule efficient room-temperature phosphorescent material
An aromatic amine and reaction technology, which is applied in the field of organic luminescence and its anti-counterfeiting, can solve the problems of difficult mass preparation, few molecular species, long synthesis steps, etc., and achieves the effects of cheap raw materials, high luminous efficiency, and simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The reaction equation is as follows:
[0037]
[0038] The specific steps of the reaction are as follows:
[0039] A mixture of compound formula A-1 and compound formula B-1 in glacial acetic acid was refluxed for 4 hours. The solid was separated from the mixture (the reaction liquid was cooled to room temperature, and the precipitate was filtered), and the product formula I-1 was obtained.
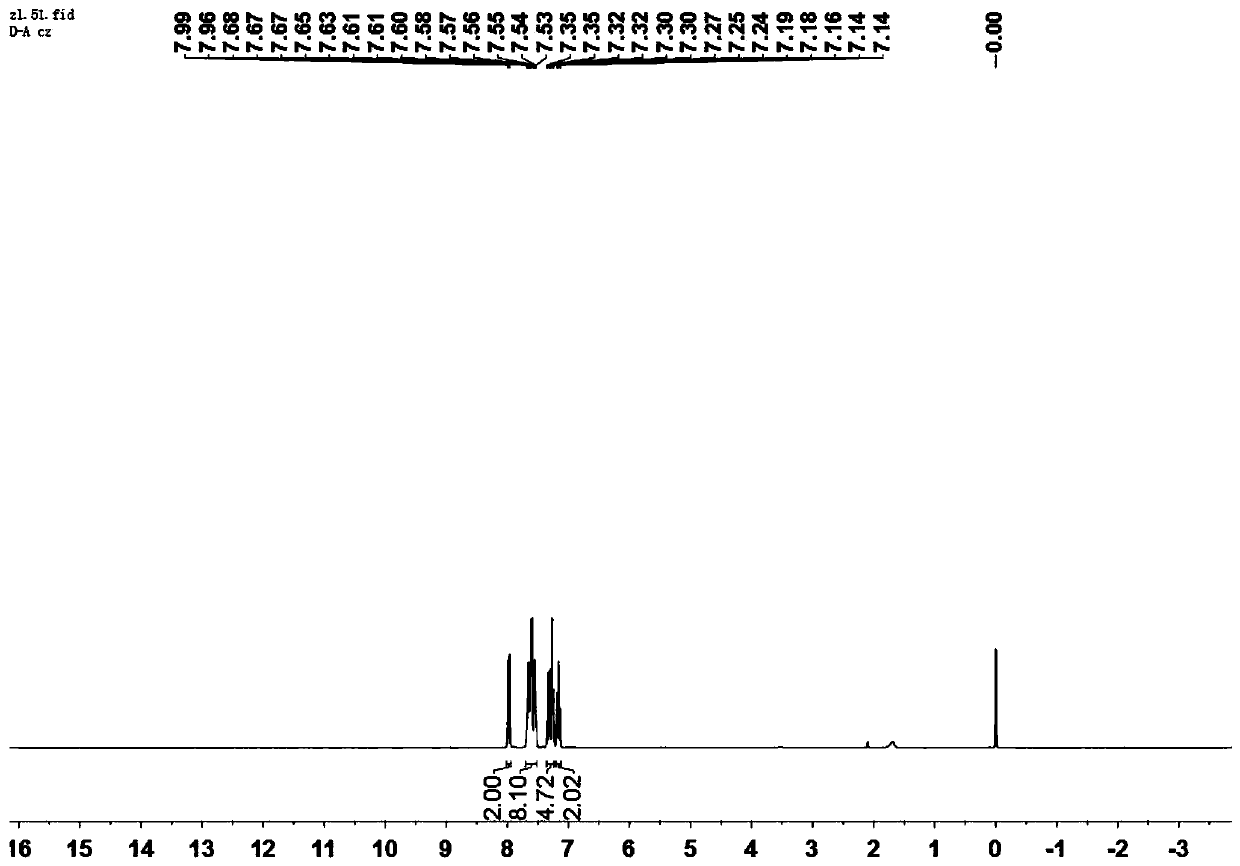
[0040] The H NMR spectrum of the compound shown in formula Ⅰ-1 is as follows: figure 1 As shown, the characterization data are as follows:
[0041] 1 H NMR (300MHz, Chloroform-d) δ7.98 (d, J=7.7Hz, 2H), 7.70–7.51 (m, 8H), 7.36–7.23 (m, 4H), 7.20–7.12 (m, 2H), HR-MS(APCI)m / z calcd for C 26 h 16 N 2 o 2 [M+H] + 389.12, found 389.13.
[0042] From the above detection results, it can be seen that the structure of the compound represented by formula I-1 is correct.
Embodiment 2
[0044] The reaction equation is as follows:
[0045]
[0046] The specific steps of the reaction are as follows:
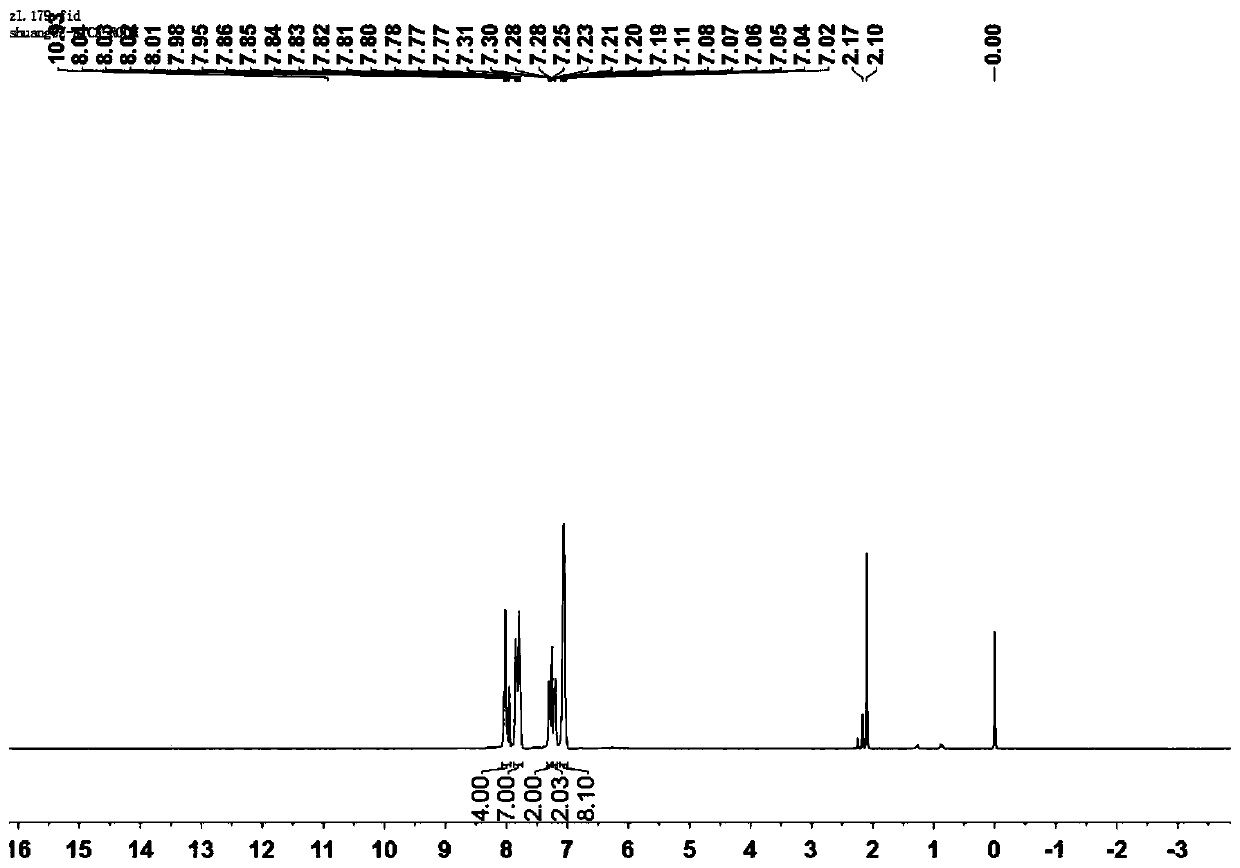
[0047] A mixture of compound formula A-1 and compound formula B-1 in glacial acetic acid was refluxed for 4 hours. The solid was separated from the mixture (the reaction liquid was cooled to room temperature, and the precipitate was filtered), and the product formula I-2 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com