Adsorbent for efficiently removing arsenite ions from water, and preparation method thereof
An arsenite and adsorbent technology, applied in chemical instruments and methods, adsorbed water/sewage treatment, water pollutants, etc., can solve the problem of low removal efficiency of arsenite ions, and achieve fast adsorption speed and good adsorption. The effect of performance, excellent adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
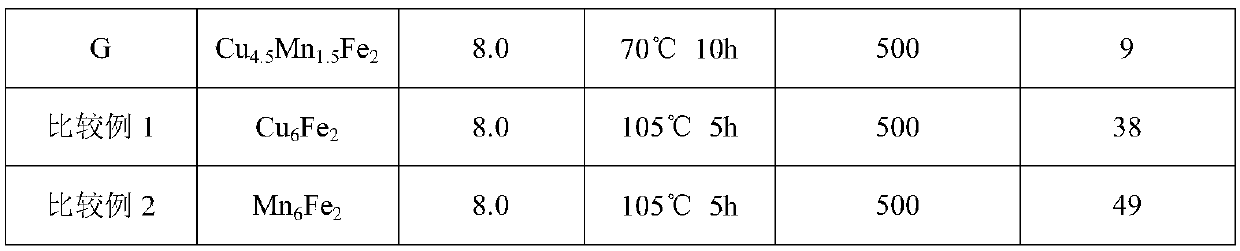
[0034] Dissolve copper nitrate, manganese nitrate and ferric nitrate in deionized water at a molar ratio of 4.5:1.5:2, stir at room temperature until clear, and record it as solution A; dissolve sodium hydroxide in deionized water, stir at room temperature until clear , recorded as solution B; quickly drop solution B into solution A, stop when the pH value of the solution reaches 8.0, continue to stir for 2 hours, pour the solution into a crystallization kettle and treat it with hydrothermal treatment at 105°C for 5 hours, filter, wash, and dry at 100 ℃ drying for 10h to obtain the adsorbent product A.
[0035] Add 12g of adsorbent A into 1L aqueous solution containing 500mg of arsenite ion at 20°C, stir for 20min and then detect the content of arsenite ion in the water. The results are listed in Table 1.
Embodiment 2
[0037] Dissolve copper nitrate, manganese nitrate and ferric nitrate in deionized water at a molar ratio of 3:3:2, stir at room temperature until clear, and record it as solution A; dissolve sodium hydroxide in deionized water, stir at room temperature until clear , recorded as solution B; quickly drop solution B into solution A, stop when the pH value of the solution reaches 8.0, continue to stir for 2 hours, pour the solution into a crystallization kettle and treat it with hydrothermal treatment at 105°C for 5 hours, filter, wash, and dry at 100 ℃ drying for 10h to obtain the adsorbent product B.
[0038] Add 12g of adsorbent B into 1L of aqueous solution containing 500mg of arsenite ions at 20°C, stir for 20 minutes and then detect the content of arsenite ions in the water. The results are listed in Table 1.
Embodiment 3
[0040] Dissolve copper nitrate, manganese nitrate and lanthanum nitrate in deionized water at a molar ratio of 4.5:1.5:2, stir at room temperature until clear, and record it as solution A; dissolve sodium hydroxide in deionized water, stir at room temperature until clear , recorded as solution B; quickly drop solution B into solution A, stop when the pH value of the solution reaches 8.0, continue to stir for 2 hours, pour the solution into a crystallization kettle and treat it with hydrothermal treatment at 105°C for 5 hours, filter, wash, and dry at 100 C and dried for 10 hours to obtain the adsorbent product C.
[0041] Add 12 g of adsorbent C to 1 L of aqueous solution containing 500 mg of arsenite ions at 20°C, stir for 20 min and then detect the content of arsenite ions in the water. The results are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com