A kind of asymmetric preparation method of florfenicol intermediate cyclic compound
A technology of florfenicol and cyclic compound, which is applied in the field of asymmetric preparation of florfenicol intermediate cyclic compound, can solve the problems of short reaction time, cannot be promoted, cannot have higher yield reaction time and the like, and achieves the The effect of easy operation, saving of raw materials and good preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
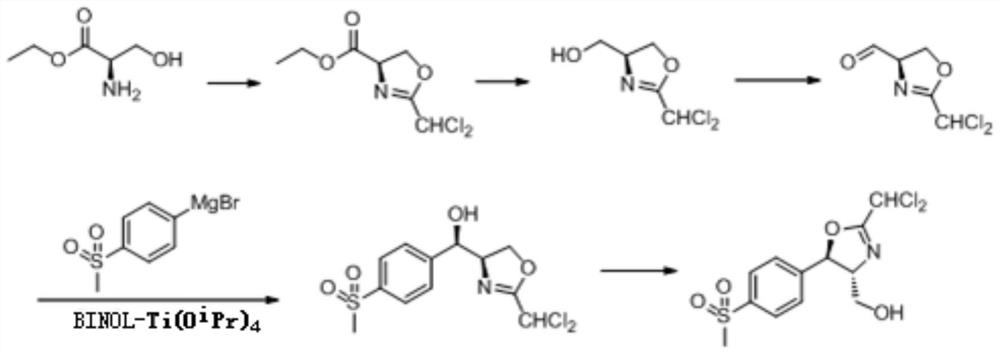
[0023] The specific preparation steps are as follows: 1) Cyclization step: D-serine ethyl ester I is selected as raw material A, 2,2-dichloromethyl imidate is selected as raw material B, dichloromethane is selected as solvent C; the molar ratio of raw material A and raw material B is The ratio is 1:1.5, and the quality of solvent C is 16 times of the total mass of raw material A and raw material B; raw material A and raw material B are added in solvent C, and sodium ethylate is selected from the base D group to add, and guarantee the content of the base material If the amount is excessive, stir the reaction at a speed of 2-5r / s at a temperature between -55 and -35°C for at least 12 hours. TLC confirms that the conversion is complete, and distills off methanol under reduced pressure. Use an appropriate amount of deionized water and The dichloromethane was repeatedly extracted several times, the organic phases were combined, dried over anhydrous sodium sulfate, and the dichlorome...
Embodiment 2
[0029] The specific preparation steps are as follows: 1) Cyclization step: D-serine ethyl ester I is selected as raw material A, 2,2-dichloromethylimidate is selected as raw material B, tetrahydrofuran is selected as solvent C; the molar ratio of raw material A and raw material B is The ratio is 1:1.2, and the quality of solvent C is at least 18 times the total mass of raw material A and raw material B; raw material A and raw material B are added to solvent C, and diisopropylethylamine is selected from base D group to add, And ensure that the amount of the alkali substance is excessive, at a temperature between -50 and -30°C, stir the reaction at a speed of 4-7r / s for at least 12h, TLC confirms that the conversion is complete, distills off the methanol under reduced pressure, and uses An appropriate amount of deionized water and dichloromethane were repeatedly extracted several times, the organic phases were combined, dried over anhydrous sodium sulfate, and the dichloromethane...
Embodiment 3
[0035] The preparation steps are as follows: 1) Cyclization step: D-serine ethyl ester hydrochloride is selected as raw material A, 2,2-dichloromethyl imidate is selected as raw material B, and chloroform and isopropanol are selected as solvent C The ratio is 1:1; the molar ratio of raw material A to raw material B is 1:1, and the mass of solvent C is at least 20 times the total mass of raw material A and raw material B; adding raw material A and raw material B to solvent C, Select triethylamine from the base D group to add, and ensure that the amount of the base is excessive, at a temperature between -45 and -25°C, stir the reaction at a speed of 6-9 / s for at least 12h, TLC Confirm that the conversion is complete, remove methanol by distillation under reduced pressure, repeatedly extract several times with appropriate amount of deionized water and methylene chloride, combine the organic phases, dry over anhydrous sodium sulfate, remove methylene chloride by distillation under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com