High-efficiency catalyst for preparing 1,3-butadiene by using carbon dioxide to oxidize 1-butene to dehydrogenate and preparation method thereof
A carbon dioxide and catalyst technology, applied in the field of high-efficiency catalysts and their preparation, can solve the problems of catalyst activity, low selectivity, poor stability, etc., and achieve the effects of abundant pores, good electrical conductivity, and avoiding agglomeration and sintering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0033] A single iron element is loaded on the activated carbon carrier. The specific preparation method is as follows:
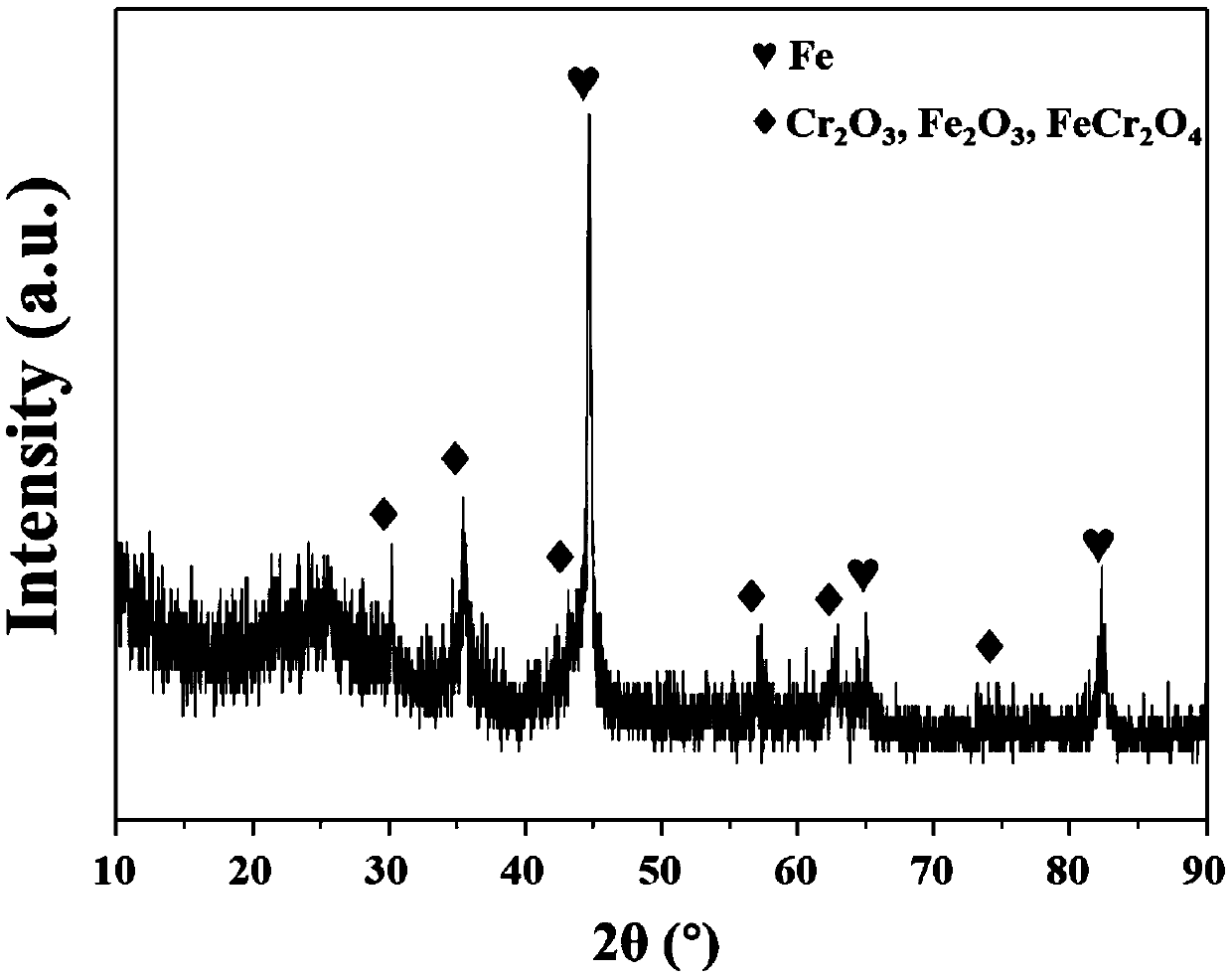
[0034] Weigh 3g of activated carbon, Fe(NO 3 ) 3 ·9H 2 O 1.0821g, that is, the loading amount of Fe (mass of iron element / mass of carrier activated carbon) is 5wt%, measure 100 mL of distilled water, and place it in an eggplant-shaped flask at 60° C. for continuous stirring for 4 hours. After the water solvent was removed by rotary evaporation, it was dried in an oven at 120°C for 4 hours. Put the above sample in the tube furnace N 2 The samples are calcined at 600℃ for 4h (heating rate 3℃ / min), which are marked as FeO X / AC.
Embodiment 2~5
[0036] Under the condition that the other experimental conditions are exactly the same as in Example 1, the Fe(NO 3 ) 3 ·9H 2 The O mass is changed to 2.1643g, 3.2464g, 4.3286g, 5.4105g. That is, the loading amount of the modulating catalyst is 10wt%, 15wt%, 20wt%, and 25wt% respectively. The optimal catalyst loading can be obtained by comparing the activity test.
Embodiment 6~10
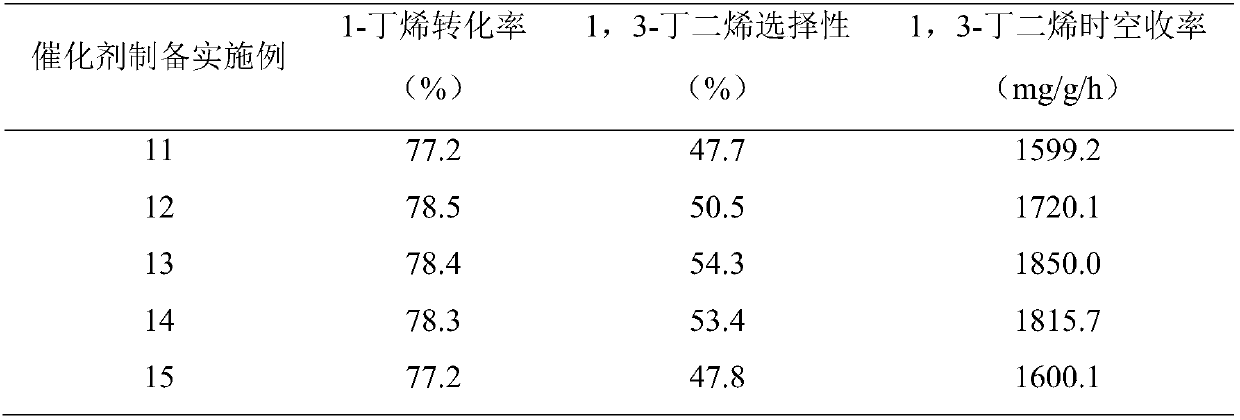
[0038] The catalyst activity is evaluated in an atmospheric micro-reaction system, with 6mL / min 1-butene and 54mL / min CO as reactants 2 , The intake air ratio CO 2 / C 4 H 8 =9:1, respectively use 0.2g of the catalyst of Examples 1-5, that is, the space velocity is 4.5h -1 , The reaction was carried out at 600°C and atmospheric pressure, and the product was analyzed by gas chromatography. Taking the conversion rate of 1-butene when the reaction is carried out for 10 minutes, the selectivity of 1-butene to 1,3-butadiene and the space-time yield of 1,3-butadiene are indicators. The obtained reaction performance is shown in Table 1. . From the results, it can be concluded that the 1,3-butadiene space-time yield of the catalyst is the highest when the iron loading is 15wt%, which is 849.1mg / g / h. Therefore, 15wt% is preferably the optimal loading of iron.
[0039] Table 1 Catalysts catalyze CO 2 The result of the dehydrogenation of 1-butene oxide to 1,3-butadiene
[0040]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Empty yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com