A kind of analysis method of compatibility between midazolam and production system
A midazolam and analysis method technology, applied in the field of analysis and detection, can solve the problems of uneven digestion, large error, small sampling amount and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
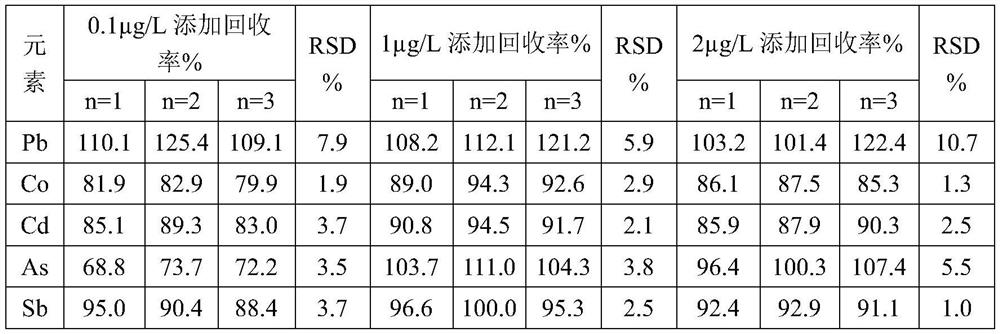
[0054] Embodiment 1 provides a kind of midazolam and the analysis method of production system compatibility, comprises the following steps:
[0055] S1: Preparation of sample master solution
[0056] Weigh 2g of midazolam, add 28mL of 1wt% dilute hydrochloric acid, concentrated nitric acid, 30wt% hydrogen peroxide in a weight ratio of 7:4:1, stir and dissolve; weigh 16g of sodium chloride, dissolve it with 1600mL of water Blank solution; add midazolam solution to the blank solution, add 5wt% diisopropylamine to it, stir well, adjust pH to 3.3 with 1% dilute hydrochloric acid solution or 0.02M sodium hydroxide solution, add water to the mark.
[0057] S2: Preparation of sample solution
[0058] Take 5mL of sample mother solution in a 50mL measuring bottle, dilute to the mark with diluent 2% nitric acid, and shake well.
[0059] S3: Preparation of blank solution
[0060] Take 5mL of water in a 50mL volumetric flask, dilute to the mark with diluent, and shake well.
[0061] S...
Embodiment 2
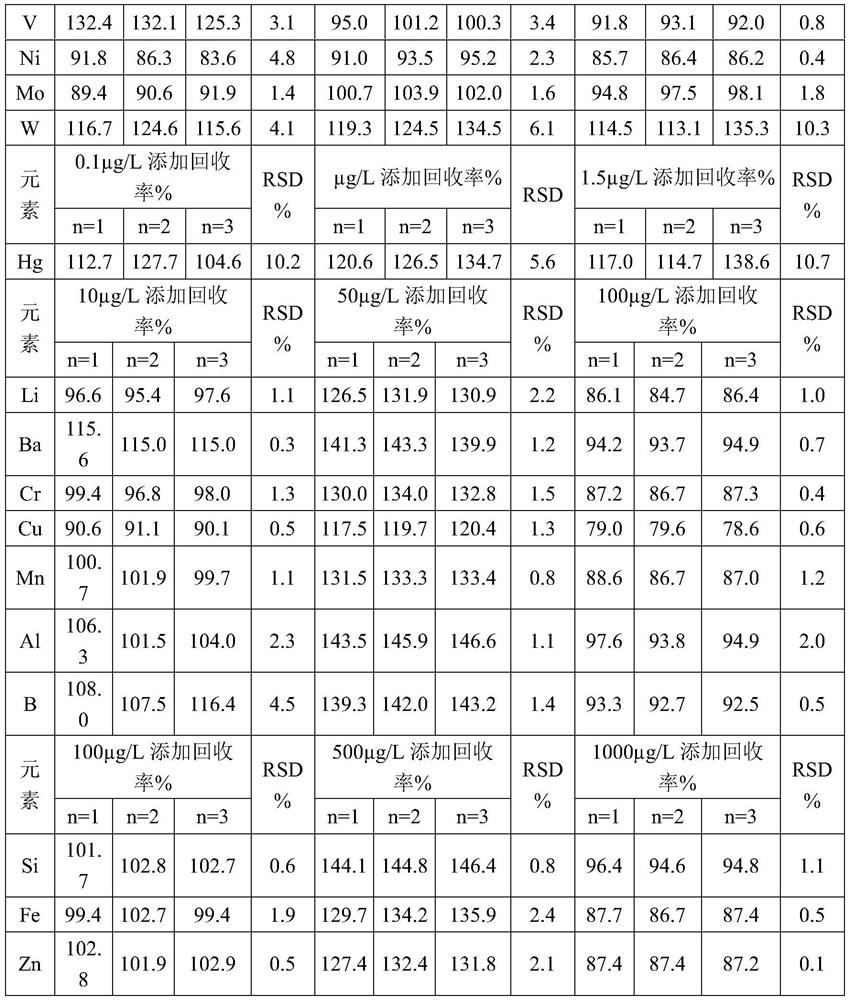
[0070] Embodiment 2 provides a kind of midazolam and the analysis method of production system compatibility, comprises the following steps:
[0071] S1: Preparation of sample master solution
[0072] Weigh 2g of midazolam, add 28mL of 1wt% dilute hydrochloric acid, concentrated nitric acid, 30wt% hydrogen peroxide in a weight ratio of 7:4:1, stir and dissolve; weigh 16g of sodium chloride, dissolve it with 1600mL of water Blank solution; add midazolam solution to the blank solution, add 1wt% diisopropylamine to it, stir well, adjust pH to 3.2 with 1% dilute hydrochloric acid solution or 0.02M sodium hydroxide solution, add water to the mark.
[0073] S2: Preparation of sample solution
[0074] Take 5mL of sample mother solution in a 50mL measuring bottle, dilute to the mark with diluent 2% nitric acid, and shake well.
[0075] S3: Preparation of blank solution
[0076] Take 5mL of water in a 50mL volumetric flask, dilute to the mark with diluent, and shake well.
[0077] S...
Embodiment 3
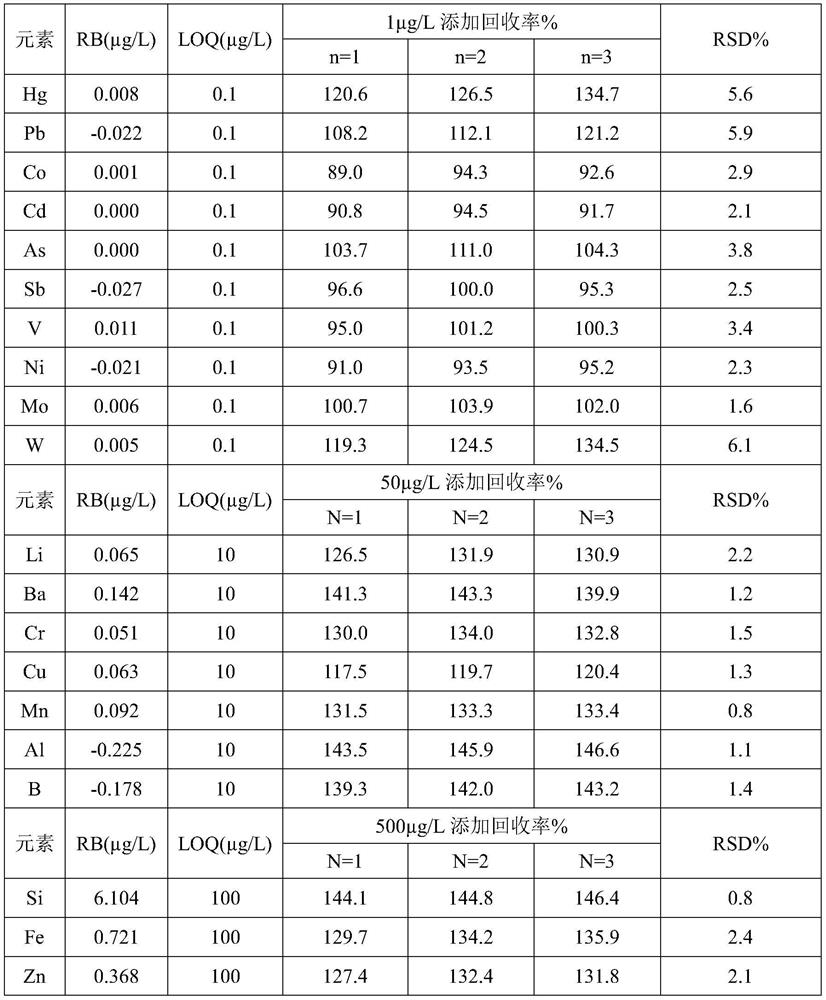
[0086] Embodiment 3 provides a kind of midazolam and the analysis method of production system compatibility, comprises the following steps:
[0087] S1: Preparation of sample master solution
[0088] Weigh 2g of midazolam, add 28mL of 1wt% dilute hydrochloric acid, concentrated nitric acid, 30wt% hydrogen peroxide in a weight ratio of 7:4:1, stir and dissolve; weigh 16g of sodium chloride, dissolve it with 1600mL of water Blank solution: add the midazolam solution to the blank solution, add 10wt% diisopropylamine thereinto, stir evenly, adjust the pH to 3.4 with 1% dilute hydrochloric acid solution or 0.02M sodium hydroxide solution, and add water to the mark.
[0089] S2: Preparation of sample solution
[0090] Take 5mL of sample mother solution in a 50mL measuring bottle, dilute to the mark with diluent 2% nitric acid, and shake well.
[0091] S3: Preparation of blank solution
[0092] Take 5mL of water in a 50mL volumetric flask, dilute to the mark with diluent, and shake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com