Application of snase in preparation of medicine for treating inflammatory bowel disease
An inflammatory bowel disease and drug technology, applied in pharmacy and medical-related fields, can solve the problems of high recurrence rate, adverse reactions, poor patient compliance, etc. The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of SNase oral preparation
[0023] Mix 57ml of span 85 and 22.8ml of absolute ethanol into a round-bottomed flask, stir magnetically in a water bath at 40°C for 10min, then add 8ml of 0.25% sodium alginate solution (drug loading 5mg SNase) dropwise, continue stirring for 10min, then Pump out the water until it becomes clear, pour 1ml of calcium chloride ethanol solution (0.1%) into it, and continue pumping and stirring for 30 minutes to solidify the nanoparticles until there are no bubbles. Centrifuge the mixed solution at 8000rpm for 30min, collect the precipitate, wash twice with petroleum ether, collect the precipitate, resuspend the precipitate with distilled water, and ultrasonically disperse with an ultrasonic cell pulverizer, 400W, 50 times / min to obtain SNase nanoparticle suspension liquid.
Embodiment 2
[0024] Example 2: Pharmacodynamic evaluation of SNase oral preparation against inflammatory bowel disease.
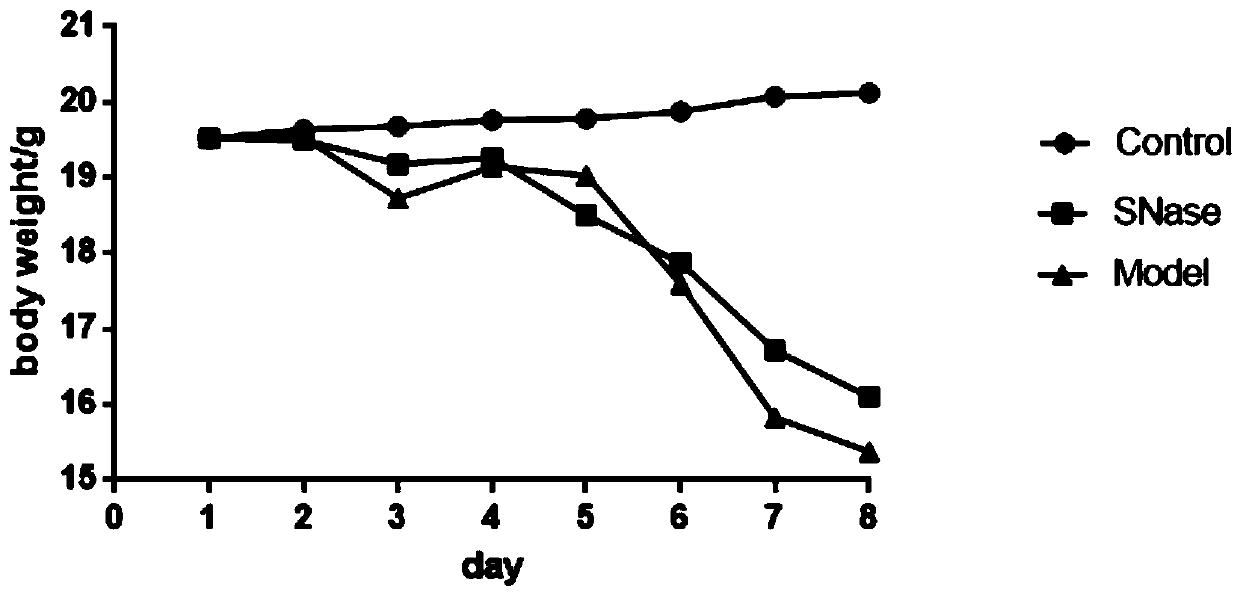
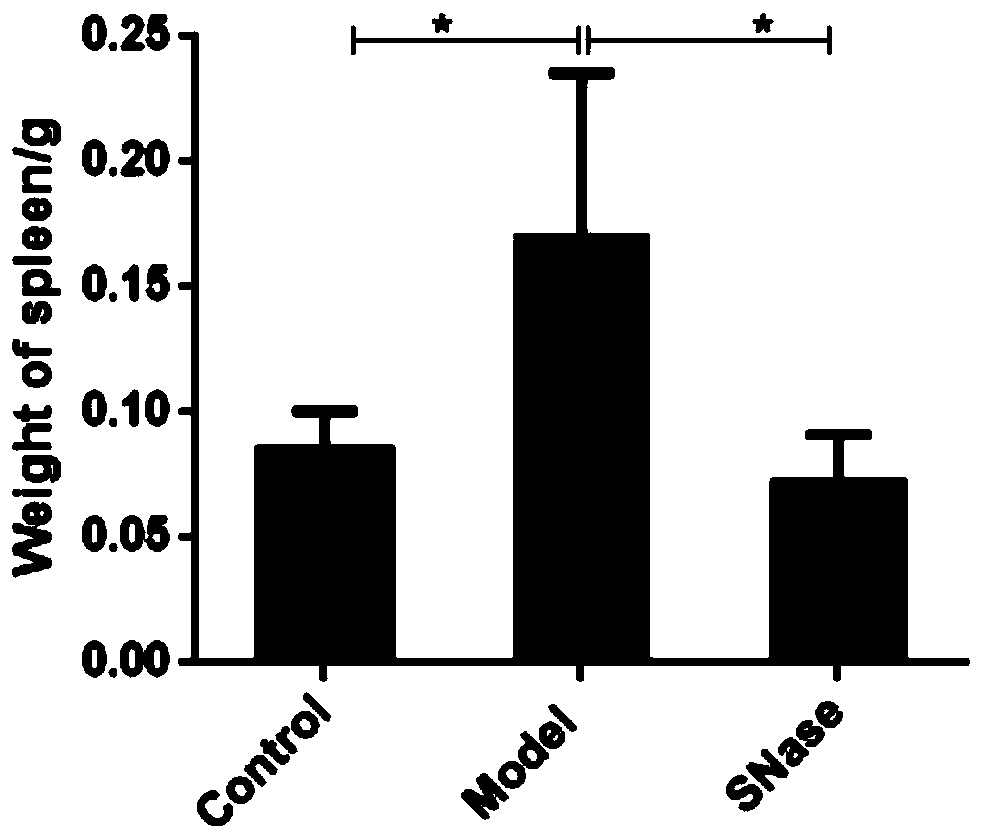
[0025] Twenty-one 8-week-old female C57BL / 6J mice (purchased from Qinglongshan Animal Breeding Factory, Jiangning District, Nanjing, license number: SCXK (Su) 2017-0001) were randomly divided into 3 groups, which were the blank group and the model group. group and the calcium alginate immobilized SNase group (i.e., the nanoparticle suspension prepared in Example 1), 7 mice in each group, and adaptively fed for three days. The solvent is made into 3% DSS (dextran sulfate sodium), so that the mice drink water freely, and embodiment 1 obtains the SNase nanoparticle suspension SNase (2.5mg / ml) group begins intragastric administration on the day of modeling, each The mice were given 200ul orally, and the model was established for 7 days, once a day, and the mice were killed by cervical dislocation on the eighth day, and the pharmacodynamics score was performed.
[0026] (1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com