Androgen receptor antagonist, its preparation method and application
A reaction and compound technology, applied in the field of medicine, can solve problems such as low biological activity and increased metabolic burden of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
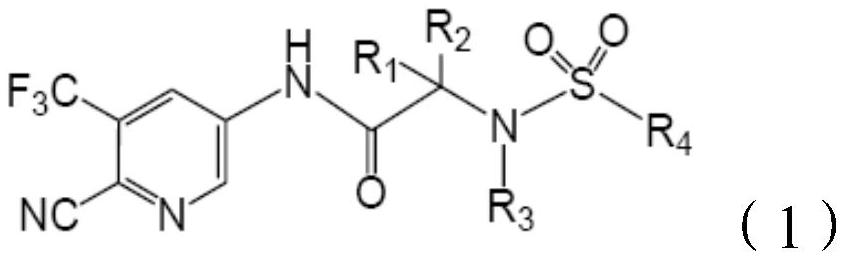
[0043] The compounds of the present invention can be produced by the following methods in addition to the production methods described above.
[0044]
[0045] where R 1 , R 2 , R 3 and R 4 The definition is the same as above, R 5 For tert-butyl or methyl.
Embodiment 1
[0047] Embodiment 1 (preparation of compound JD1001-2053)
[0048]
[0049] Add 5.0g (15.4mmol, 1.0eq) Fmoc-1-aminocyclopropanecarboxylic acid to a 200mL three-necked flask under nitrogen protection, then add 50mL of DCM, 4 drops of DMF, and add 9.8g of oxalyl chloride (76.9 mmol, 5.0eq), stirred and reacted at room temperature for 4 hours, took out two drops of the reaction solution and added methanol to treat it as methyl ester, detected the completion of the reaction by TLC (developing solvent: PE:EA=1:1), and distilled off the oxalyl chloride under reduced pressure and DCM to obtain a pale yellow solid, which was stored under nitrogen gas for subsequent use.
[0050] Take the light yellow solid obtained in the previous step reaction, add 90mL dry THF, 1.9g (22.6mmol, 1.5eq) NaHCO 3 , 3.4g (18.3mmol, 1.2eq) 5-amino-3-trifluoromethyl-2-cyanopyridine, heated to 60°C, maintained the temperature and stirred for 2 hours, and the reaction was completed by TLC detection (devel...
Embodiment 2
[0053] Embodiment 2 (preparation of compound JD1001-2054)
[0054]
[0055] Add JD1001-002-9 solid 0.10g (0.37mmol, 1.0eq), THF 3.2mL, DMAP 0.14g (1.11mmol, 3.0eq), 3,5-difluorobenzenesulfonate into a 25mL round bottom flask under nitrogen protection Acyl chloride 0.24g (1.11mmol, 3.0eq), stirred and reacted at room temperature for 3 hours, the completion of the reaction was detected by TLC (developing solvent: PE:EA=1:1), adding saturated NaHCO 3 Quench the reaction with aqueous solution, extract with ethyl acetate to obtain the organic phase, wash the organic phase twice with 0.5N hydrochloric acid aqueous solution, dry over anhydrous sodium sulfate, filter, concentrate the organic phase to obtain a solid, and prepare the liquid phase (CH 3 CN:H 2 O=60:40) separation and purification, and about 24 mg of the product JD1001-2054 was obtained by freeze-drying, with a yield of 16%.
[0056] 1 H NMR: (DMSO,ppm,400MHz)δ1.07(2H,m),1.40(2H,m),7.51(2H,m),7.57(1H,m),8.60(1H,d),8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com