Docetaxel-cyclosporine A co-encapsulated self-emulsifying preparation and preparation method thereof
A docetaxel, self-emulsifying technology, applied in pharmaceutical formulations, emulsion delivery, non-active components of polymer compounds, etc. to improve bioavailability, improve dissolution, and improve tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
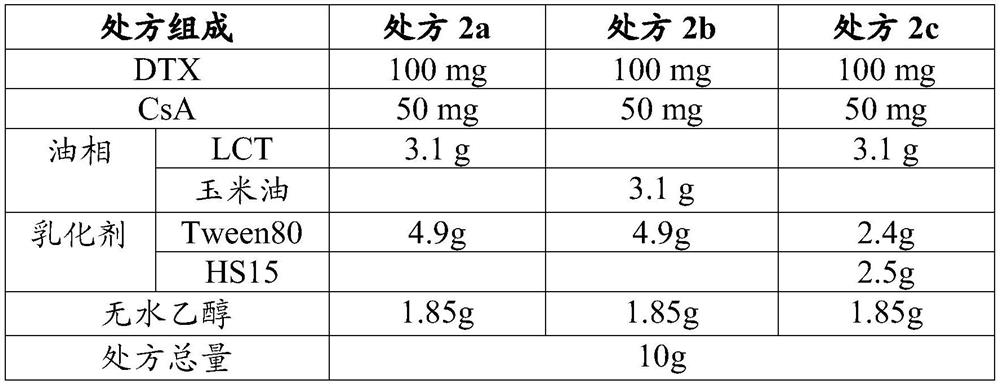
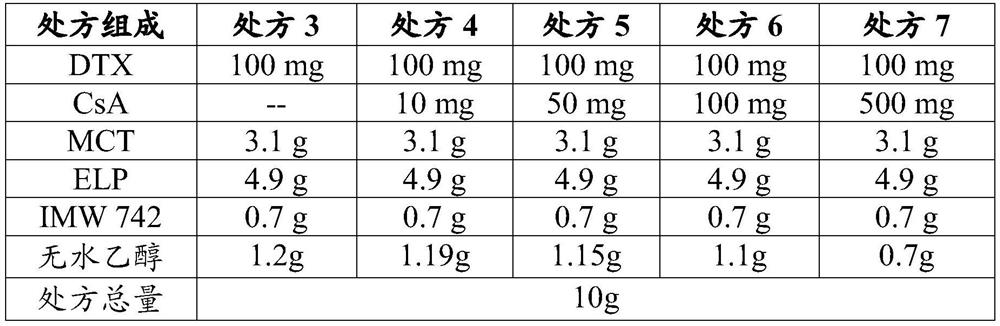
[0078] prescription:
[0079] DTX 1g CsA 0.5g MCT 31g ELP 49g IMW 742 7g Absolute ethanol 11.5g total prescription 100g
[0080] Weigh docetaxel and cyclosporine A according to the above prescription, dissolve them completely with absolute ethanol, then add medium-chain triglycerides, polyoxyethylene 35 castor oil, caprylic capric acid mono-diglyceride, Stir well, dispense and seal with a gland.
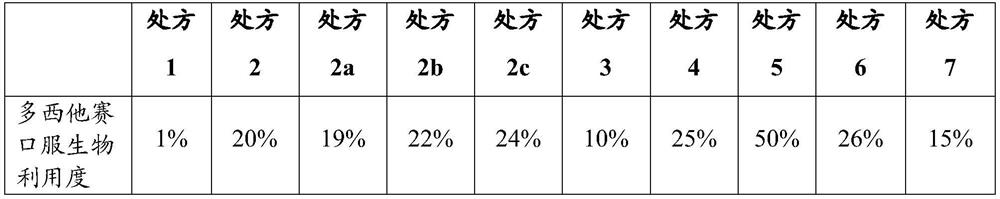
[0081] The oral bioavailability of docetaxel of the obtained preparation was measured by the method described in the above test example 2, and the result was 52.5%.
Embodiment 2~8
[0083] Using the same method as in Example 1, the preparations of Examples 2 to 8 were prepared according to the prescriptions in Table 9, and the oral bioavailability of docetaxel was determined, as shown in Table 9.
[0084] Table 9
[0085]
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com