Non-catalytic efficient preparation method of two-dimensional fluorine-containing covalent organic framework compound
A covalent organic framework and compound technology, which is applied in the field of non-catalytic and efficient preparation of two-dimensional fluorine-containing covalent organic framework compounds, can solve the problems of long reaction time, difficulty in synthesizing crystalline products, and the need for catalysts, etc., and achieve a simple reaction process , high yield, enhanced electrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
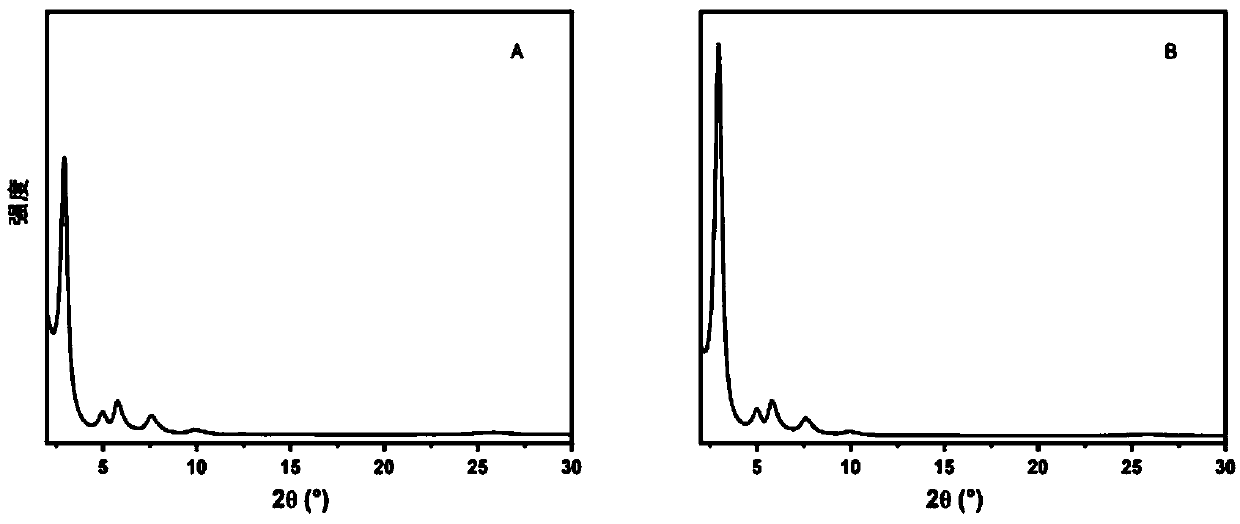
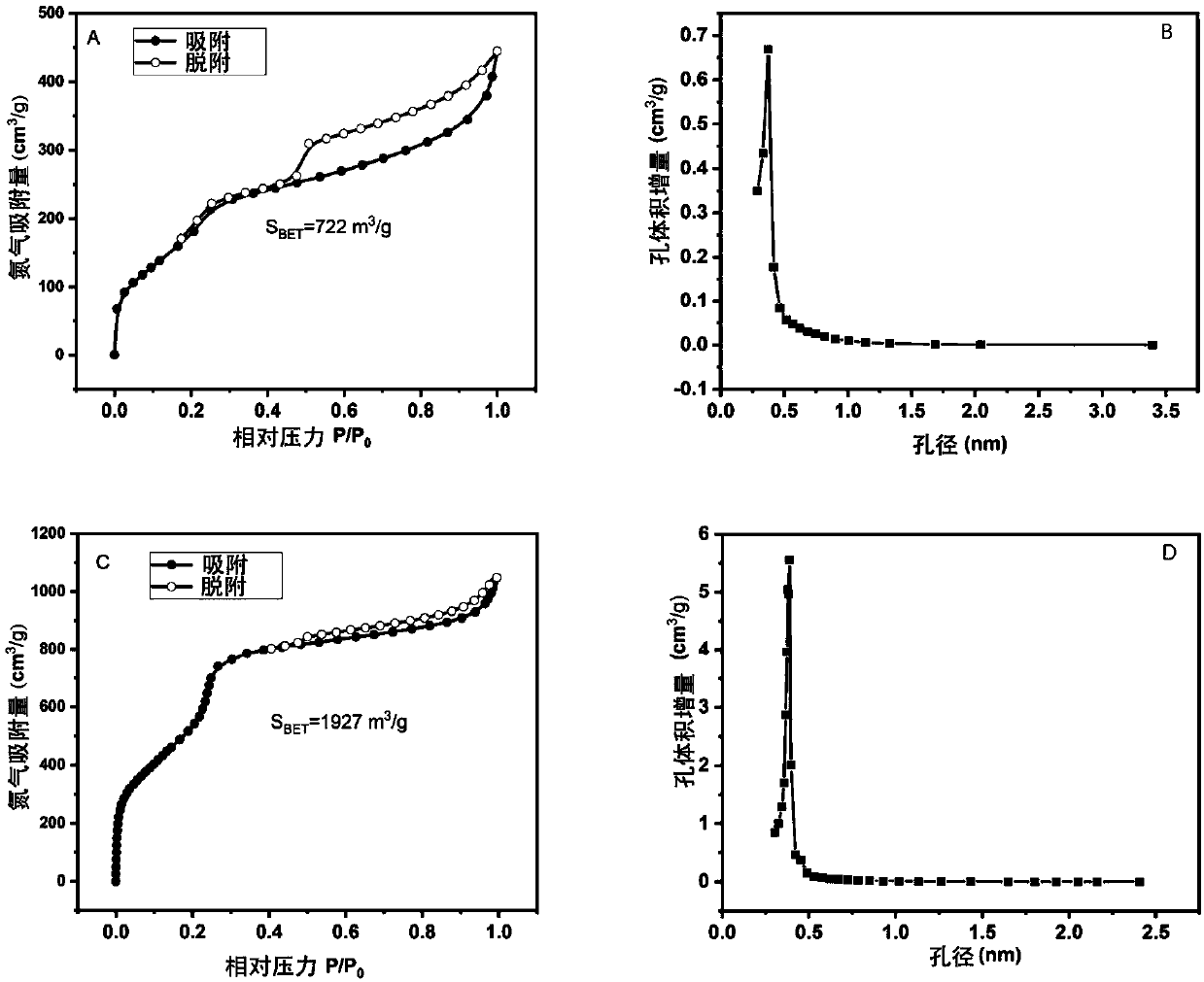
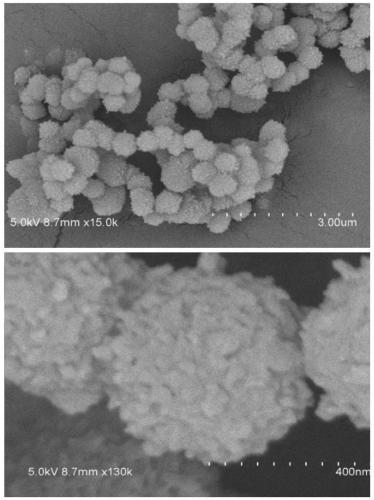
[0034] A non-catalytic and efficient preparation method of a two-dimensional fluorine-containing covalent organic framework compound, comprising the following steps:
[0035] 1) Weigh 2,3,5,6-tetrafluoroterephthalaldehyde (82.4mg) and 2,4,6-tris(4-aminophenyl)-1,3,5 with a molar ratio of 1:1.5 - Triazine (94.4mg) in a 2mL heat-resistant glass tube, add 1mL dioxane / mesitylene (v / v=1:1) solvent;
[0036] 2) Freeze the glass tube with liquid nitrogen, evacuate to 0 mbar for three minutes, degas, repeat three times, and seal the vacuum glass tube; ultrasonicate the glass tube for 20 minutes, put it in a 120°C oven for 5 minutes, and obtain the crude product;
[0037] 3) The crude product was taken out and filtered, washed with tetrahydrofuran solution, drained, wrapped with clean filter paper, put into a Soxhlet extractor, and washed with tetrahydrofuran solution at 100°C for 12 hours under reflux;
[0038] 4) Take out the product after reflux washing, and put it into the separat...
Embodiment 2
[0040] A non-catalytic and efficient preparation method of a two-dimensional fluorine-containing covalent organic framework compound, comprising the following steps:
[0041] 1) Weigh 2,3,5,6-tetrafluoroterephthalaldehyde (82.4mg) and 2,4,6-tris(4-aminophenyl)-1,3,5 with a molar ratio of 1:1.5 - Triazine (94.4mg) in a 2mL heat-resistant glass tube, add 1mL dioxane / mesitylene (v / v=1:1) solvent;
[0042] 2) Freeze the glass tube with liquid nitrogen, evacuate to 0 mbar for three minutes, degas, repeat three times, and seal the vacuum glass tube. Sonicate the glass tube for 20 minutes, and put it in a 120°C oven for 24 hours to obtain the crude product;
[0043] 3) The crude product was taken out and filtered, washed with tetrahydrofuran solution, drained, wrapped with clean filter paper, put into a Soxhlet extractor, and washed with tetrahydrofuran solution at 100°C for 12 hours under reflux;
[0044] 4) Take out the product after reflux washing, and put it into the separation...
Embodiment 3
[0053] A non-catalytic and efficient preparation method of a two-dimensional fluorine-containing covalent organic framework compound, comprising the following steps:
[0054] 1) Weigh 2,3,5,6-tetrafluoroterephthalaldehyde (82.4mg) and 2,4,6-tris(4-aminophenyl)-benzene (93.6mg) with a molar ratio of 1:1.5 In a 2mL heat-resistant glass tube, add 1mL of dioxane / mesitylene (v / v=1:1) solvent;
[0055] 2) Freeze the glass tube with liquid nitrogen, evacuate to 0 mbar for three minutes, degas, repeat three times, and seal the vacuum glass tube. The glass tube was sonicated for 20 minutes, and placed in an oven at 180° C. for 1 minute to obtain a crude product.
[0056] 3) The crude product was taken out and filtered, washed with tetrahydrofuran solution, drained, wrapped with clean filter paper, put into a Soxhlet extractor, and washed with tetrahydrofuran solution at 100°C for 12 hours under reflux;
[0057] 4) Take out the product after reflux washing, and dry it (put it in an ov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com