Preparation method of fluoroacetamide hapten and application of monoclonal antibody
A technology of fluoroacetamide and hapten, which is applied in the field of preparation of fluoroacetamide hapten and monoclonal antibody, can solve problems that hinder the application and promotion of immunological methods, difficult immunological detection methods, and lack of immunogenicity. Achieve the effect of good application prospect, high practical value and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
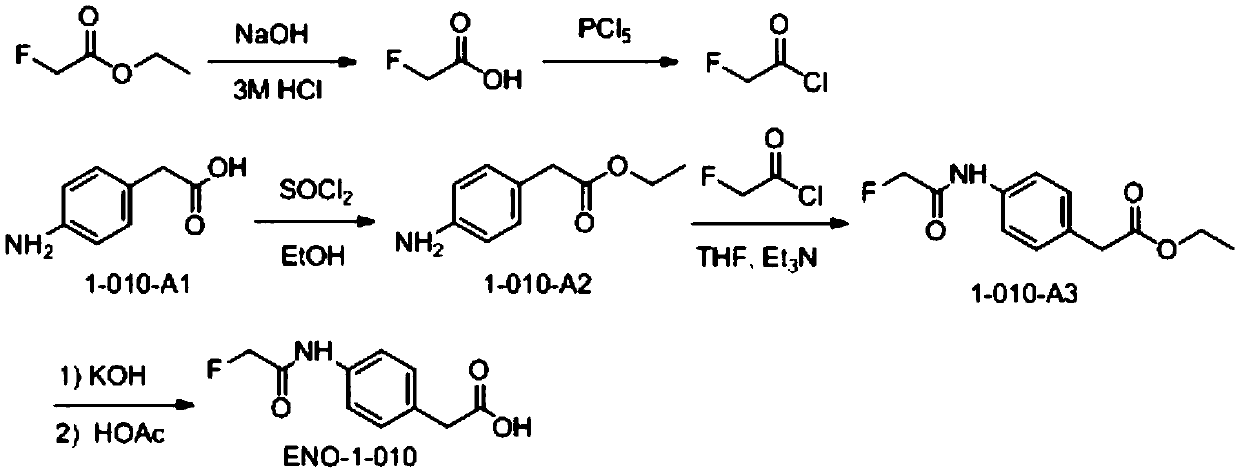
[0080] Example 1 Fluoroacetamide hapten (fluoroacetamide phe) and its synthesis and identification
[0081] 1. Synthesis of fluoroacetamide phe (such as figure 1 shown)
[0082] 1) Synthesis of fluoroacetyl chloride
[0083] An aqueous solution (34 mL) of KOH (19 g, 0.339 mol) was slowly added dropwise to ethyl fluoroacetate (30 g, 0.283 mol) in ethanol (300 mL) at room temperature, forming a white precipitate. The mixture was stirred overnight at room temperature (30-35°C). The solvent was spin-dried, and the obtained sodium salt was redissolved in hydrochloric acid (3M, 200mL), and NaCl solid was added to make the solution saturated, then extracted with ether for 4 to 5 times, the combined organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was spun After drying, fluoroacetic acid (15 g) was obtained.
[0084] In the three-neck flask with a thermometer and a condenser tube inserted, add PCl 5 (44g, 0.211mol, 1.1eq), cooled in an ice bath to...
Embodiment 2
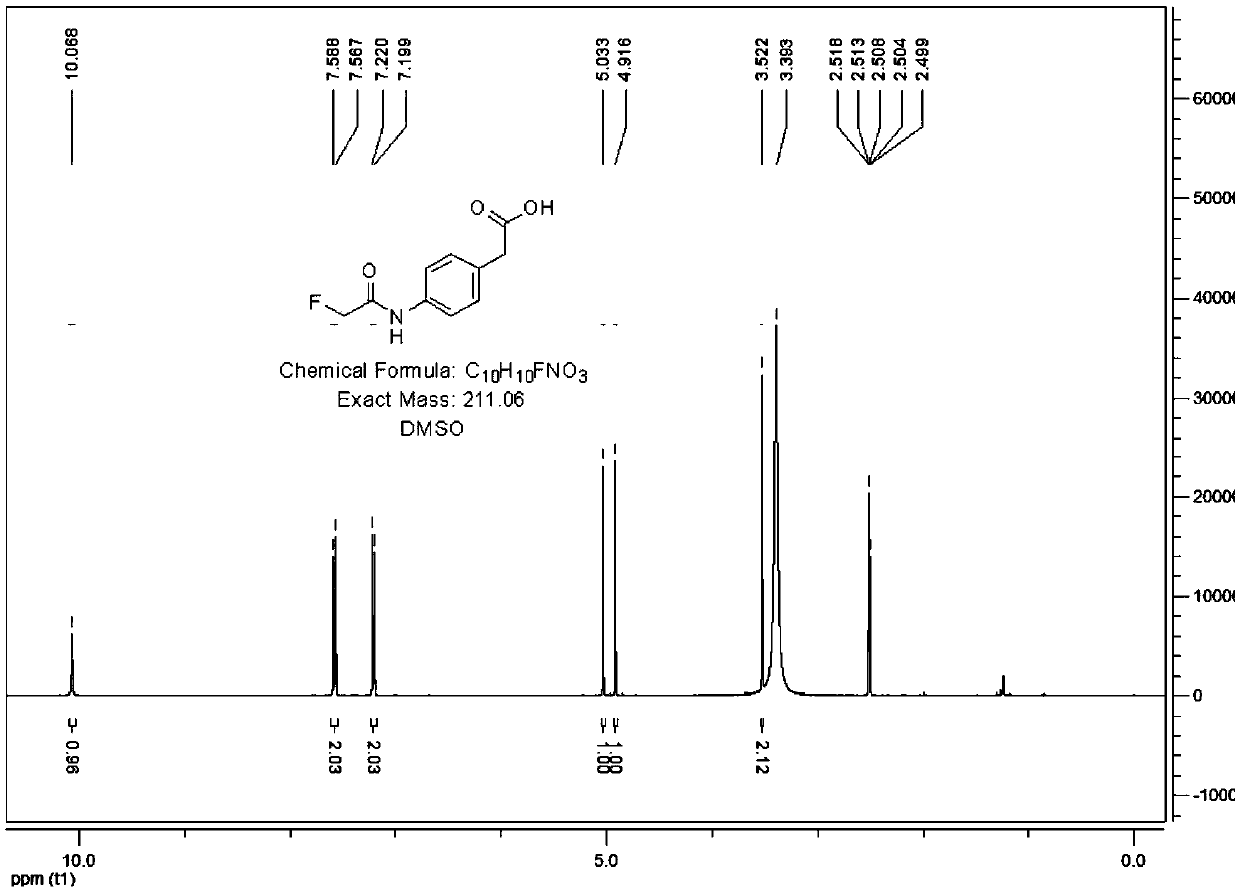
[0095] Example 2 Fluoroacetamide hapten (fluoroacetamide liner) and its synthesis and identification
[0096] 1. Synthesis of fluoroacetamide liner (such as figure 2 shown)
[0097] 10g (76mmol) of 6-aminocaproic acid was added to 100ml of anhydrous methanol, the solution was cloudy, 23g (227mml) of triethylamine was added, stirred at room temperature for 10min, 12g (113mmol) of ethyl fluoroacetate was added dropwise, the reaction was refluxed overnight, and the solution Become clear, cool to room temperature, add acetic acid to adjust the pH to 5-6, concentrate the reaction solution, drain the oil pump to obtain an oily liquid, add EtOAc to dissolve, wash EA twice with saturated saline, dry, spin dry, and drain the oil pump to obtain The product is 7g, and the yield is about 48%.
[0098] 2. Identification of fluoroacetamide liner
[0099] Its structure was determined by proton NMR spectroscopy:
[0100] 1 H NMR (400MHz, CDCl 3 )δ: 6.48(bs,1H,NH); 4.87(s,1H,CH 2 ); 4....
Embodiment 3
[0102] Example 3 Preparation of fluoroacetamide artificial antigen
[0103] 1. Preparation and identification of fluoroacetamide coating agent
[0104] 1.1 Preparation of fluoroacetamide coating agent
[0105] 50mg of fluoroacetamide hapten (formula II or III) dissolved in 1ml N,N-dimethylformamide (DMF), N,N'-dicyclohexylcarbodiimide (DCC) 80mg, N - 60 mg of hydroxysuccinimide (NHS) was added to the above fluoroacetamide hapten solution, stirred at room temperature for 2 hours; 160 mg of BSA was dissolved in 20 ml of PBS buffer (0.01mol / L PH=7.4), and stirred The activated drug was added dropwise to the protein solution and reacted overnight at 4°C; then the reaction solution was put into a dialysis bag, dialyzed with saline solution at 4°C for 48 hours, and the water was changed 4 times; save.
[0106] 1.2 Identification of fluoroacetamide coating agent
[0107] Fluoroacetamide artificial antigen is prepared into a solution in PBS for flight mass spectrometry analysis, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory dose | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com